| Issue |
Parasite
Volume 32, 2025
Special Issue – Ninth International Symposium on Monogenea. Invited Editors: Amit Tripathi, Nirupama Agarwal & Jean-Lou Justine
|
|
|---|---|---|
| Article Number | 30 | |
| Number of page(s) | 17 | |
| DOI | https://doi.org/10.1051/parasite/2025022 | |
| Published online | 21 May 2025 | |
Research Article
Role of Trachemys scripta elegans in polystome (Platyhelminthes, Monogenea, Polystomatidae) spillover and spillback following the trade of freshwater turtles in southern Europe and North America
Rôle de Trachemys scripta elegans dans la propagation et transmission des polystomes (Plathelminthes, Monogenea, Polystomatidae) suite au commerce de tortues d’eau douce en Europe du Sud et en Amérique du Nord
1
Université Perpignan Via Domitia, Centre de Formation et de Recherche sur les Environnements Méditerranéens, UMR 5110, 66860 Perpignan, France
2
CNRS, Centre de Formation et de Recherche sur les Environnements Méditerranéens, UMR5110, 66860 Perpignan, France
3
Unit for Environmental Sciences and Management, North-West University, Potchefstroom, 2531, South Africa
4
CTHerpConsultant, 40 Pine Street, Plantsville, CT 06479, USA
5
Davidson College, Biology Department, Box 7118, Davidson, NC 28035, USA
6
Institute of Systems Genomics, Department of Molecular and Cell Biology, University of Connecticut, Storrs, CT 06269, USA
7
South African Institute for Aquatic Biodiversity, Somerset Street, Makhanda 6139, South Africa
* Corresponding author: verneau@univ-perp.fr
Received:
26
July
2024
Accepted:
10
April
2025
The red-eared slider, Trachemys scripta elegans (Wied, 1938), has been introduced worldwide, partly because of the exotic pet trade in the 1980s and 1990s. When T. s. elegans is released or escapes into natural environments, it often establishes new feral populations due to its tolerance for a variety of aquatic ecosystems. Therefore, it is now considered one of the most invasive species in the world because it can compete with native turtle species. In the present study, our objectives were to identify the potential for polystome spillover and spillback resulting from the introduction of the red-eared slider into new environments in North America. Fieldwork investigations were thus conducted mainly in aquatic habitats in Florida and North Carolina, United States, but also in Connecticut, Indiana, Kansas, Maine, Nebraska and New York. Using DNA barcoding based on cytochrome c oxidase I (COI) sequences, we surveyed the species diversity of polystome within American freshwater turtles. These included T. s. elegans but also Apalone ferox, Apalone spinifera, Chelydra serpentina, Chrysemys picta, Kinosternon baurii, Pseudemys spp., Sternotherus minor and Sternotherus odoratus. Genetic evidence confirmed that invasive populations of T. s. elegans in southern Europe have transmitted their own polystomes to native host species following spillover effects, and revealed here that T. s. elegans in non-indigenous habitats in the United States acts as a new reservoir of infection for native polystomes following spillback effects, thus increasing indigenous parasite transmission in the wild. Together, these findings raise further concern about the spread of non-native turtles and their impact on parasite transmission.
Résumé
La tortue à tempes rouges, Trachemys scripta elegans (Wied, 1938), a été introduite dans le monde entier, en partie grâce au commerce d’animaux de compagnie exotiques dans les années 1980 et 1990. Lorsque T. s. elegans est relâchée ou s’échappe dans des milieux naturels, elle établit souvent de nouvelles populations sauvages suite à sa tolérance à une variété d’écosystèmes aquatiques. De ce fait, elle est aujourd’hui considérée comme l’une des espèces les plus invasives au monde, car elle peut concurrencer les espèces de tortues indigènes. Dans cette étude, nos objectifs étaient d’identifier le potentiel de propagation et de transmission des polystomes résultant de l’introduction de la tortue de Floride à tempes rouges dans de nouveaux environnements en Amérique du Nord. Des campagnes de terrain ont donc été menées principalement dans les habitats aquatiques de Floride et de Caroline du Nord aux États-Unis, mais aussi dans le Connecticut, l’Indiana, le Kansas, le Maine, le Nebraska et l’État de New York. En utilisant le code-barre ADN basé sur les séquences de la cytochrome c oxydase I (COI), nous avons étudié la diversité des espèces de polystomes chez les tortues d’eau douce américaines. Celles-ci comprenaient T. s. elegans mais aussi Apalone ferox, Apalone spinifera, Chelydra serpentina, Chrysemys picta, Kinosternon baurii, Pseudemys spp., Sternotherus minor et Sternotherus odoratus. Les preuves génétiques ont confirmé que les populations invasives de T. s. elegans en Europe du Sud ont transmis leurs propres polystomes aux espèces hôtes indigènes suite à des effets de débordement, et ont révélé ici que T. s. elegans dans les habitats non indigènes des États-Unis agit comme un nouveau réservoir d’infection pour les polystomes indigènes suite à des effets boule de neige, augmentant ainsi la transmission des parasites indigènes dans la nature. Ces résultats suscitent de nouvelles inquiétudes quant à la propagation des tortues non indigènes et à leur impact sur la transmission du parasite.
Key words: Trachemys scripta elegans / Parasites / Polystomes / Invasions / Reservoir host / Spillover event / Spillback event
Edited by: Jean-Lou Justine.
© O. Verneau et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Vector-borne diseases of humans, livestock and wildlife are transmitted by diverse invertebrate hosts, including mosquitoes, sandflies, ticks, flies, fleas, lice and aquatic snails [16, 46]. Vectors typically act as carrier hosts where pathogens multiply and develop before being transmitted to another definitive or intermediate host, mostly actively through direct contact although other processes are sometimes considered [53]. Besides the significant role played by vectors in disease transmission, numerous animals comprising fish, amphibians, reptiles, birds and mammals can also act as disease reservoirs for transmitting parasites to other animals [21]. If reservoir hosts serve as healthy carriers of parasites, once transmitted to other host competent species, parasites can potentially cause significant zoonotic health issues. This was illustrated in numerous cases following handling of wild animals. For example, it is now well known that salmonellosis is associated with exotic pets, especially iguanas and turtles [52, 54]. Between 1996 and 1997 in the United States, there were 74,000 Salmonella infections that were associated with reptile or amphibian contact [34], and strong correlations were observed between salmonellosis and turtle handling during Salmonella outbreaks [19, 20]. Whereas the presence of Salmonella has been detected in free living native turtles across natural wetland environments of Southwestern Europe, it was also documented in the same environments from feral populations of the common slider Trachemys scripta (Thunberg, 1792), as well as from pet turtles. This demonstrates that feral populations of T. scripta acting as animal reservoirs may represent an additional risk factor for Salmonella or some other parasite infections for humans and native animals [24, 33]. This is why host–parasite interactions involving T. scripta should be investigated more in depth in the environments where sliders were introduced.
With just over 200 species reported, polystomes (Platyhelminthes, Monogenea, Polystomatidae) infect semi-aquatic vertebrates, including the Australian lungfish Neoceratodus forsteri (Krefft, 1870), amphibians, freshwater turtles and the common hippopotamus, Hippopotamus amphibius Linnaeus, 1758 [12]. These parasites, which are globally distributed, are recovered mostly from the gills and/or the urinary bladder of amphibians, and from the urinary bladder, pharyngeal cavity, or conjunctival sacs of freshwater turtles, while they are reported from gills and skin of lungfishes and from conjunctival sacs of hippopotamuses. They all display a direct life cycle with no intermediate host, which involves mature parasites that produce and release eggs at different rates depending of the type of host, host reproductive status, host behaviour and external temperature (see Du Preez et al. [12]). Once eggs are released into the water, ciliated larvae develop and hatch usually within two to three weeks. After contact with a suitable host, larvae use different migration routes on the host depending on the polystome genus. Larvae of Apaloneotrema, Aussietrema and Fornixtrema migrate to the conjunctival sacs, while larvae of Polystomoidella and Uropolystomoides enter via the cloaca before establishing in the urinary bladder. Lastly, larvae of Manotrema and Uteropolystomoides establish directly in the mouth and pharyngeal pouches (see Du Preez et al. [12]). Because Pleurodirotrema and Polystomoides both include species that infect either the urinary bladder or the oral region of their hosts [5, 10], they complete the two distinct life cycles as described above depending on the parasite’s ecological niche. While polystomes can be regarded mostly as host- and site-specific parasites (see Du Preez et al. [12] for a review), some polystomes that infect turtles have also been reported from several host species living in outdoor turtle enclosures at zoological aquariums or gardens, turtle farms or private properties [51]. For example, this is the case for Polystomoides coronatus (Leidy, 1888 [30]), which was originally described from the pharyngeal cavity of an unidentified American turtle. According to Price [41], this parasite infected the urinary bladder of the red-eared slider Trachemys scripta elegans (Wied, 1838) and the pharyngeal cavity of the spiny softshell turtle Apalone spinifera (Lesueur, 1827) at the zoological aquarium of New York City, NY. This is also the case for Polystomoides orbicularis (Stunkard, 1916 [43]), which was formerly described from the urinary bladder of the painted turtle Chrysemys picta (Schneider, 1783). This parasite was also reported to infect the pharyngeal cavity of the Florida softshell turtle Apalone ferox (Schneider, 1783) and the urinary bladder of the Cumberland slider Trachemys scripta troosti (Holbrook, 1836), T. s. elegans and C. picta at the zoological aquarium of New York City [41]. In the early 2010s, Polystomoides oris Paul, 1938 [36], which was originally described from the pharyngeal cavity of C. picta in aquatic ecosystems in the United States, and P. orbicularis were both recorded from European turtles including the Mediterranean pond turtle Mauremys leprosa (Schweigger, 1812) and the European pond turtle Emys orbicularis (Linnaeus, 1758) in outdoor turtle enclosures in southern France [51]. Overall, these results suggested that polystomes of turtles were not as strictly host-specific as originally hypothesized, at least in zoological parks where turtles can be found in the same artificial pools.
These conclusions were further supported by studies of polystome biodiversity within populations of native freshwater turtles in natural aquatic environments across Europe. With the exception of the native polystome species, namely Polystomoides euzeti (Combes & Ktari, 1976 [7]) and Polystomoides tunisiensis Gonzales & Mishra 1977 [17], which infect the urinary bladder and the pharyngeal cavity of M. leprosa, respectively, and Polystomoides ocellatus (Rudolphi, 1819 [42]), which is found in the pharyngeal cavity of E. orbicularis, all other polystomes found within both European turtles originated from North American turtles [22, 35]. Because non-native polystomes were also found within feral populations of T. s. elegans across European wetlands, it was concluded that these turtles may carry alien polystomes in southern Europe [22, 35] and transmit their parasites to indigenous turtles following spillover events.
These results thus raised important questions about the role of T. s. elegans as a disease reservoir following spillover or spillback events. These events are thought to account for non-indigenous parasite dispersal and indigenous parasite dynamics, respectively, following introduction of non-native host species into new environments [8, 9, 21, 28, 40]. In other words, the introduced host acts as a reservoir of infection for non-native parasites in spillover, whereas it acts as a new reservoir of infection for native parasites in spillback [28]. Therefore, to address the role of the red-eared slider in polystome dispersal and transmission, we studied the polystome diversity among turtles in American freshwater ecosystems, with a particular focus on T. s. elegans. Because the red-eared slider was also introduced to several regions of the United States outside its native range [50] (Fig. 1), we expected to find greater species richness of polystomes within native freshwater turtles in America in areas where they now co-occur with T. s. elegans. Cytochrome c oxidase I (COI) sequences used as DNA barcodes were then obtained from polystome eggs and/or specimens that were collected from several species of turtles during three decades of fieldwork along aquatic ecosystems in the United States (Connecticut, Florida, Indiana, Kansas, Maine, Nebraska, New York and North Carolina). The biodiversity of polystomes that was inferred from the survey of T. s. elegans in its native as well as in non-native areas of American and non-American freshwater environments was compared to that of other sympatric, or even syntopic, turtle species. Turtles concerned A. ferox, A. spinifera, the common snapping turtle Chelydra serpentina (Linnaeus, 1758), C. picta, the false map turtle Graptemys pseudogeographica (Gray, 1831), the striped mud turtle Kinosternon baurii (Garman, 1891), the eastern river cooter Pseudemys concinna (Le Conte, 1830), the Florida cooter Pseudemys floridana (Le Conte, 1830), the Florida redbelly turtle Pseudemys nelsoni Carr, 1938, the peninsula cooter Pseudemys peninsularis Carr, 1938, the loggerhead turtle Sternotherus minor (Agassiz, 1857) and the common musk turtle Sternotherus odoratus (Latreille, 1802) from North America, but also M. leprosa and E. orbicularis from Europe. Our results reinforce prior observations that T. s. elegans acts as a reservoir host for spillover of polystomes outside the United States. They shed further light on a parasite spillback effect by T. s. elegans, which could have significant consequences for native turtles in America and the dynamics of aquatic ecosystems worldwide.
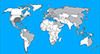 |
Figure 1 Schematic representation of the T. s. elegans range distribution according to Van Dijk et al. [50]. Original range in dark grey; range following human translocation in light grey. |
Materials and methods
Ethics
All turtles mentioned in this study were examined taking into account local laws and regulations, and non-invasive techniques were used as much as possible to obtain the parasites without killing the hosts. Ethical clearance was obtained from the North-West University Animal Care ethics committee (Ethical clearance no. NWU-00256-17A5). Permits were obtained from the North Carolina Wildlife Resources Commission and the Connecticut Department of Energy & Environmental Protection for collection in 2015 (Permit numbers 15-SC01038, 18-SC01287 & 1515004).
Areas of investigation
In the United States, T. scripta (T. s. elegans and T. s. scripta) was collected from freshwater ecosystems of Indiana and Kansas where it occurs naturally without human intervention, and in Florida, Maine and North Carolina where it was introduced. Chrysemys picta was sampled in Connecticut, Indiana, New York and North Carolina, C. serpentina in Nebraska and North Carolina, A. ferox and Pseudemys spp. in Florida and A. spinifera with G. pseudogeographica in Indiana. In Europe, T. s. elegans, M. leprosa and E. orbicularis were surveyed mainly in the southwestern and southeastern parts of France and in the northern part of Spain. Mauremys leprosa and E. orbicularis were also surveyed in Morocco and Algeria, as T. s. elegans does not occur there. Some turtles were also inspected for parasites in non-natural aquatic environments, especially through outdoor turtle enclosures of the turtle farm of Sorède in southern France, and to a lesser extent through outdoor turtle enclosures of two private properties in France, namely at La Sauzière-Saint-Jean and Béziers [51]. Turtles collected in artificial ornamental ponds and settling basins for wastewater were also examined.
Host and polystome sampling
Fieldwork investigations were conducted towards the end of the 1990s to 2018, usually from early spring to end of summer in freshwater aquatic environments. Sites of interest were ponds, lakes and rivers (Table 1). Turtles were captured with traps that were baited with fish or pork liver, set in water bodies for one to several days and checked daily for the presence of turtles [22, 23, 35]. Turtles were also collected by hand or with the help of landing nets in outdoor turtle enclosures. After capture, turtles were individually placed in plastic containers with clean water to cover about half the turtle’s body for one to three days. Water was then filtered every day through a pair of soil sieves with mesh size 500 and 100 micron, respectively [51], and examined for the presence of polystome eggs. Collected eggs were preserved in 70% molecular grade ethanol for later molecular analysis. Worms were also collected following the dissection of several infected turtles when it was not feasible to extract adult polystomes without killing animals or simply to identify the location of parasites in the host. Some specimens were preserved in 10% buffered formalin for further biometric and morphological analyses, while others were stored in 70% molecular grade ethanol for DNA barcoding.
Geographical areas of American and French aquatic environments investigated for turtles and their polystomes.
Molecular and phylogenetic analyses
DNA was extracted from eggs and/or adult worms following the procedure described in Verneau et al. [51]. Partial COI was amplified using the combination of either Forward L-CO1p (5′–TTTTTTGGGCATCCTGAGGTTTAT–3′) and Reverse H-Cox1p2 (5′–TAAAGAAAGAACATAATGAAAATG–3′) primers [31] or Forward L-CO1p and Reverse HCOX1R (5′–AACAACAAACCAAGAATCATG–3′) primers [35]. Amplification and sequencing procedures were reported elsewhere in Héritier et al. [22], Meyer et al. [35] and Verneau et al. [51]. After inspection of chromatograms with SeqScape v2.5 software (Applied BioSystems, Waltham, MA, USA), sequences obtained in this study were aligned with all previously published COI sequences [5, 14, 22, 23, 35, 51], using Clustal W [47] implemented in MEGA6 [45]. Identical sequences were subsequently grouped into unique haplotypes, which were further analyzed with the help of MrBayes software [25].
Using Modeltest 3.06 [39], a GTR + I + Γ model was selected for running the Bayesian analysis. We defined three partitions according to codon positions 1, 2 and 3, and parameters for the selected GTR + I + Γ model were estimated independently for each partition (see Héritier et al. [22]). After running four chains of ten million generations each, which were sampled every 100 cycles, Bayesian posterior probabilities were estimated after removing the first 10,000 trees as the burn-in phase. The tree was rooted with sequences of two polystomes infecting amphibians, namely Wetapolystoma almae Gray, 1993 [18] and Polystoma naevius Caballero & Cerecero 1941 [4]. The final 50% majority consensus tree was then visualized with FigTree v.1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) and depicted as a radial tree to show phylogenetic relationships of all haplotypes and as a phenogram to depict phylogenetic relationships between all distinct species.
Host and polystome species identification
Host species identity was mainly based on external morphological characters which permit turtle identification. When uncertainty was encountered for some specimens, the cytochrome b was sequenced and resulting nucleotide sequences were compared to sequence databases with BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for species recognition. Concerning polystome species identity, whereas morphological characters are usually inadequate or not sufficiently informative for species recognition within a genus, morphometrics based on reproductive and fixing organs from mature worms are usually satisfactory for species determination [12]. However, morphometrics cannot be assessed from eggs collection. This is why polystome species delimitation was done here solely from COI DNA barcoding. Based on a cumulative error plot that was inferred from K2P COI distances within three polystomes, i.e. P. oris, P. tunisiensis and P. euzeti, Héritier et al. [22] found a large gap between intra and interspecific genetic divergences. Accordingly, they estimated that the probability of committing type I errors was less than 0.05 for co-specific specimens assuming an optimum threshold of 3.4% of genetic divergence. Therefore, species delimitation within polystomes was done according to the COI threshold designed by Héritier et al. [22].
Results
The American freshwater turtles that were investigated for polystomes included A. ferox, A. spinifera, C. picta, C. serpentina, G. pseudogeographica, K. baurii, Ps. concinna, Ps. floridana, Ps. nelsoni, Ps. peninsularis, S. minor, S. odoratus and T. scripta. Prevalence of infection is summarized in Table 2 and in [5] for each turtle species according to their location. A total of 63 new sequences (accession numbers PQ052824 to PQ052886 in GenBank) were generated in this study from polystome eggs and/or adults that were collected in 2015 and 2018 (Table 3), characterizing 27 distinct haplotypes. When adding them to sequences extracted from GenBank, the COI haplotypic diversity for chelonian polystomes segregated into 134 distinct haplotypes (see Online Resource) whose phylogenetic relationships are depicted in Figure 2. Based on that tree and the COI threshold defined by Héritier et al. [22], 37 chelonian polystomes could be considered, whose relationships are depicted in Figure 3 after collapsing haplotypes referring to the same species. In total, we identified 22 polystome species infecting native and/or non-native American freshwater turtles, i.e., eight from the conjunctival sacs, five from the urinary bladder, seven from the oral cavity and two from undetermined sites of infection (Table 4). Among these species, 13 are still presumptively undescribed based on the COI threshold designed for polystomes.
 |
Figure 2 Radial tree depicting the phylogenetic relationships of the 134 discrete COI chelonian polystome haplotypes after Bayesian analysis. |
 |
Figure 3 Phylogram of chelonian polystome species derived from the tree depicted in Figure 2 after collapsing haplotypes referring to the same species. Polystome species in blue refer to polystomes infecting the conjunctival sacs, in red the urinary bladder and in green the oral cavity of American turtles. Polystomatidae sp1 and sp2, which also infect American turtles, were not highlighted as the infection site was not determined. |
Polystome samples (worms and/or eggs) for which partial COI sequences were obtained in this study.
Polystome species and haplotypic diversity among American (Apalone ferox, A. spinifera, Chelydra serpentina, Chrysemys picta, Graptemys pseudogeographica, Kinosternon baurii, Pseudemys concinna, P. floridana, P. nelsoni, P. peninsularis and Trachemys scripta) and Mediterranean (Emys orbicularis and Mauremys leprosa) turtle species across natural North American and Mediterranean freshwater environments, as well as in outdoor turtle enclosures.
The polystome diversity of T. s. elegans currently stands at 12 species, considering that host sampling was conducted across freshwater ecosystems in the United States, but also in France and Spain where the red-eared slider was introduced. These species were Fornixtrema elizabethae (Platt, 2000 [38]), Fornixtrema spA, Fornixtrema spB, P. orbicularis, P. oris, Polystomoides scriptanus Héritier et al., 2018 [23], Polystomoides soredensis Héritier et al., 2018 [23], Polystomoides sp2, Polystomoides sp3, Polystomoides sp4, Polystomatidae sp1 and Polystomatidae sp2. Additionally, there were two polystomes associated with A. ferox, namely Apaloneotrema moleri (Du Preez & Morrison 2012 [13]) and Polystomoides rugosus (MacCallum, 1918 [32]), a single polystome associated with A. spinifera, namely Polystomoides sp1, three polystomes associated with C. serpentina, namely F. elizabethae, Fornixtrema spA and Fornixtrema spF, three polystomes associated with C. picta, namely F. elizabethae, P. orbicularis and P. oris, a single polystome with G. pseudogeographica, namely Fornixtrema spB, three polystomes associated with K. baurii, namely Fornixtrema spE, Polystomoidella whartoni Price 1939 [41] and Polystomoidella sp1, three polystomes with Ps. concinna, namely Fornixtrema spC, Fornixtrema spD and Uteropolystomoides multifalx (Stunkard, 1924 [44]), a single polystome with Ps. floridana, namely U. multifalx, two polystomes with Ps. nelsoni, namely Fornixtrema spC and U. multifalx, and three polystomes with Ps. peninsularis, namely Fornixtrema spC, Fornixtrema spD and P. scriptanus (Table 4).
Discussion
The ecological role of T. s. elegans in polystome spreading across European freshwater ecosystems potentially showing a spillover effect
Regarding the polystome diversity within T. s. elegans across European natural freshwater ecosystems and in outdoor turtle enclosures – nine species in total – it should be noted that all but one, Polystomatidae sp2 of the red-eared slider, were shared with M. leprosa and E. orbicularis in their native ranges (underlined polystome species in Table 4). Another one, i.e., F. elizabethae, was also shared between T. s. elegans and M. leprosa but never reported from T. s. elegans in European freshwater environments. Considering that these parasites originated from American turtles, Héritier et al. [22] and Meyer et al. [35] hypothesized that host switching may have occurred from T. s. elegans to both European turtles, either in the wild, when turtles were found sympatrically in the same habitats, or following the release or translocation of native turtles after they became infected in outdoor turtle enclosures. This conclusion was also supported by the lack of T. s. elegans across freshwater ecosystems of Algeria and Morocco and the absence of its polystomes through M. leprosa and E. orbicularis in these environments. Héritier et al. [22] and Meyer et al. [35] thus considered that T. s. elegans could serve as a carrier of alien parasites in natural environments. Conversely, the native polystomes of M. leprosa, namely P. euzeti and P. tunisiensis, and those of E. orbicularis, namely P. ocellatus, were never reported within T. s. elegans, whether in natural environments or in confined environments such as outdoor turtle enclosures. Therefore, T. s. elegans may serve as a reservoir host for spillover of non-indigenous polystomes across turtles in European freshwater environments, but does not appear to act as a new reservoir host for native polystomes in the same environments.
As a result, this raises numerous questions about the immune response of the red-eared slider to polystomes. How can we explain the susceptibility of T. s. elegans to its own polystomes, as well as to polystomes of C. picta and G. pseudogeographica for instance, while it appears to be resistant to the polystomes of E. orbicularis, which belongs to the same turtle family Emydidae, and to polystomes of M. leprosa? Polystomes found in American turtles could be less susceptible to immune defenses than are polystomes inhabiting European turtles, or simply better competitors. Regardless of the reason, T. s. elegans may then act as a sink reservoir host for European polystomes contributing to a dilution effect in their dynamics (see [6, 15]). This would explain the paucity of native polystomes, i.e., P. euzeti and P. tunisiensis in M. leprosa and P. ocellatus in E. orbicularis. Ultimately, if T. s. elegans serves as a source of non-native polystomes and as a sink for native polystomes in freshwater environments, all three native polystomes could quickly go extinct, at least across European natural environments (see also [22, 35]). While experimental infestations have been completed with polystomes of amphibians [1, 11, 26, 29] and turtles [37], cross experimental infestations with native and non-native parasites could be used to study the immune response of the host [27, 48, 49], with respect to relative competitive ability and interactions between polystomes [2, 48].
The ecological role of T. s. elegans in polystome transmission across American freshwater ecosystems potentially showing a spillback effect
While nine polystomes were recorded from either captive or feral populations of T. s. elegans across European freshwater ecosystems, only seven species were documented within red-eared sliders across American wetland environments (polystome species with an asterisk in Table 4). Among these, three species, i.e., P. scriptanus, P. soredensis and Polystomoides sp2 were reported from the pharyngeal cavity of their hosts. Polystomoides scriptanus occurred in Florida and North Carolina, P. soredensis occurred in Florida, Indiana, Maine and North Carolina, whereas Polystomoides sp2 occurred only in North Carolina. Despite the occurrence of P. scriptanus in M. leprosa from outdoor turtle enclosures and the occurrence of P. soredensis in M. leprosa and E. orbicularis from outdoor turtle enclosures and across European natural environments, T. s. elegans was considered the original host species for both polystomes by Héritier et al. [23]. Because M. leprosa and E. orbicularis are not found in American wetlands, Héritier et al. [23] considered that it was very unlikely that European turtles serve as a carrier for polystomes to the United States. One may therefore question the origin of these two distinct polystomes as well as the origin of Polystomoides sp2 in the pharyngeal cavity of the same host species across American freshwater environments. While T. s. elegans has been introduced globally into developed countries where young turtles were sold as pets, its current distribution in the United States also extends beyond its native range [3, 50]. The presence of T. s. elegans in Florida, Maine and North Carolina is also recognized as a consequence of the release of pet turtles that have become established in feral populations, while Indiana and Kansas are part of their native distribution. Considering that selection over evolutionary timescales should have led to a single polystome within each ecological niche in the host, we hypothesize that P. soredensis is a true native polystome of T. s. elegans as it is the single species infecting the pharyngeal cavity of the red-eared slider in the state of Indiana. We also assume that Polystomoides sp2 and P. scriptanus may have colonized T. s. elegans from other American turtle species. Even though the host has not yet been identified for Polystomoides sp2, it could be Ps. peninsularis for P. scriptanus, Ps. peninsularis being indeed the single American turtle species also infected by this parasite. Besides these three polystomes that were recorded from T. s. elegans across American wetland environments, P. oris was also reported from T. s. elegans, however only from European freshwater environments. Because P. oris was described early by Paul [36] from C. picta in the United States, it is very likely that P. oris infected T. s. elegans in this country from C. picta.
Two other polystomes, i.e., P. orbicularis and Polystomoides sp3, were recorded within red-eared sliders across American wetland environments, both infecting the urinary bladder of their host. Concerning Polystomoides sp3, which has not yet been described, it was only found in Indiana and Kansas, which both correspond to the native range of the red-eared slider. This species may therefore represent a tissue-specific polystome found in the urinary bladder of its host. In the same way as discussed above for P. soredensis, we may hypothesize that this innominate species is also a true native polystome for the red-eared slider. For P. orbicularis, while it was reported from T. s. elegans in Florida and North Carolina, which are two areas where the red-eared slider was introduced, it was not recorded in Indiana and Kansas. Moreover, P. orbicularis was documented within C. picta in Indiana and North Carolina. Because the painted turtle has been recognized as the original host of P. orbicularis, the occurrence of this parasite in the urinary bladder of T. s. elegans likely reflects a switch from painted turtles to red-eared sliders across American freshwater environments.
Finally, three polystomes, i.e., F. elizabethae, Fornixtrema spA and Fornixtrema spB were recorded within red-eared sliders across American wetland environments, all of them infecting the conjunctival sacs of their host. Since F. elizabethae was described from C. picta in Michigan and reported from the same host species in Wisconsin and Indiana, on the one hand [38], and because Fornixtrema spB was reported from G. pseudogeographica in Indiana, on the other (Platt, unpublished observations), it is likely that these two species switched from these two hosts to T. s. elegans. If we rely on this hypothesis, F. elizabethae may also have switched to C. serpentina. Finally, as Fornixtrema spA was also reported from C. serpentina, a switch from C. serpentina to T. s. elegans is also plausible.
As a consequence, P. soredensis and Polystomoides sp3 may represent native polystomes for T. s. elegans, infecting the pharyngeal cavity and the urinary bladder of their host, respectively, whereas P. scriptanus, Polystomoides sp2 and P. oris from the pharyngeal cavity, P. orbicularis from the urinary bladder and F. elizabethae, Fornixtrema spA and Fornixtrema spB from the conjunctival sacs may represent non-native polystomes for T. s. elegans. Therefore, if the red-eared slider acts as a reservoir host for spillover of polystomes in non-American freshwater environments, thus enhancing exotic parasite spreading (see above), given our data, T. s. elegans could act as a reservoir host for spillback of polystomes in American freshwater environments, thus increasing native parasite transmission in the wild.
Conclusion
Our results on the origin and distribution of polystomes in American and European freshwater environments still raise numerous questions, including (i) the extent of polystome diversity within T. s. elegans in natural environments in the United States, (ii) which species of American turtles served as parasite donors before polystomes were dispersed through red-eared sliders acting as reservoir hosts across European wetland ecosystems, and (iii) because T. s. elegans acts as a reservoir host for spillover of polystomes among European freshwater turtles and as a reservoir host for spillback of polystomes among American freshwater turtles, what are the genetic determinants underlying host specificity? To answer these questions, infection experiments and parasitological surveys in areas where turtle diversity is the highest and where T. s. elegans also occurs with other native species are needed. We therefore plan to conduct cross-infection experiments with native and non-native polystomes which will constitute the first stage to understand how host-specific polystomes may become generalists in this existing turtle system and help better understand the consequences of parasite invasions driven by an invasive turtle species. The absence of P. oris, for instance, within American red-eared sliders while it occurs within painted turtles in the states of Connecticut, Indiana, New York and North Carolina as well as within red-eared sliders in outdoor turtle enclosures in France and within native European turtle species across natural environments, still remains unclear.
Acknowledgments
We acknowledge the CNRS, the University of Perpignan and the South African National Research Foundation (NRF) for financial support and thank several postgraduate students and post-docs for their assistance in the field. We also thank anonymous reviewers for their helpful and constructive remarks on an earlier version of this manuscript. Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary material
Online Resource 1. COI haplotype diversity of polystomes sampled from turtles in aquatic natural environments and/or confined areas. Lines in purple indicate parasite specimens collected in outdoor turtle enclosures. Access here
References
- Badets M, Boissier J, Brémond P, Verneau O. 2009. Polystoma gallieni: Experimental evidence for chemical cues for developmental plasticity. Experimental Parasitology, 121, 163–166. [CrossRef] [PubMed] [Google Scholar]
- Badets M, Morrison C, Verneau O. 2010. Alternative parasite development in transmission strategies: how time flies! Journal of Evolutionary Biology, 23, 2151–2162. [CrossRef] [PubMed] [Google Scholar]
- Burger J. 2009. Red-eared slider turtles (Trachemys scripta elegans), Available at http://depts.washington.edu/oldenlab/wordpress/wp-content/uploads/2013/03/Trachemys-scripta-elegans_Burger.pdf. [Google Scholar]
- Caballero C, Cerecero EC. 1941. Una nueva especie de Polystoma (Trematoda: Polystomatidae) parasite de la vejiga urinaria de Hyla baudinii (Dum Y. Bibr.). Anales del Instituto de Biologia, Universidad Nacional Autónoma Mexico, 12, 621. [Google Scholar]
- Chaabane A, Du Preez L, Johnston G, Verneau O. 2022. Revision of the systematics of the Polystomoidinae (Platyhelminthes, Monogenea, Polystomatidae) with redefinition of Polystomoides Ward, 1917 and Uteropolystomoides Tinsley, 2017. Parasite, 29, 56. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Chaves LF, Hernandez M-J, Dobson AP, Pascual M. 2007. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends in Parasitology, 23, 311–316. [CrossRef] [PubMed] [Google Scholar]
- Combes C, Ktari MH. 1976. Neopolystoma euzeti n. sp. (Monogenea: Polystomatidae) premier représentant du genre Neopolystoma Price 1939 en Afrique. Annales de Parasitologie Humaine et Comparée, 51, 221–225. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science 287, 443–449. [CrossRef] [PubMed] [Google Scholar]
- Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM, Byers JE, Coon CAC, Frankel VM, Holt RD, Hufbauer RA, Kanarek AR, Schierenbeck KA, Wolfe LM, Perkins SE. 2012. Indirect effects of parasites in invasion. Functional Ecology, 26, 1262–1274. [CrossRef] [Google Scholar]
- Du Preez LH, Domingues MV, Verneau O. 2022. Classification of pleurodire polystomes (Platyhelminthes, Monogenea, Polystomatidae) revisited with the description of two new genera from the Australian and Neotropical Realms. International Journal for Parasitology: Parasites and Wildlife, 19, 180–186. [CrossRef] [Google Scholar]
- Du Preez LH, Kok DJ. 1997. Supporting evidence of host-specificity among southern African polystomes (Polystomatidae: Monogenea). Parasitology Research, 83, 558–562. [CrossRef] [PubMed] [Google Scholar]
- Du Preez LH, Landman WJ, Verneau O. 2023. Polystomatid flatworms: State of knowledge and future trends. Cham, Switzerland: Springer Nature Switzerland AG. [CrossRef] [Google Scholar]
- Du Preez LH, Morrison C. 2012. Two new polystomes (Monogenea: Polystomatidae) from the eyes of North American freshwater turtles. Zootaxa, 3392, 47–59. [Google Scholar]
- Du Preez LH, Verneau O. 2020. Eye to eye: classification of conjunctival sac polystomes (Monogenea: Polystomatidae) revisited with the description of three new genera Apaloneotrema n. g., Aussietrema n. g. and Fornixtrema n. g. Parasitology Research, 119, 4017–4031. [CrossRef] [PubMed] [Google Scholar]
- Faust CL, Dobson AP, Gottdenker N, Bloomfield LSP, McCallum HI, Gillespie TR, Diuk-Wasser M, Plowright RK. 2017. Null expectations for disease dynamics in shrinking habitat: dilution or amplification? Philosophical Transactions B, 372, 20160173. [CrossRef] [PubMed] [Google Scholar]
- Fèvre EM, Bronsvoort BM, Hamilton KA, Cleaveland S. 2006. Animal movements and the spread of infectious diseases. Trends in Microbiology, 14, 125–131. [CrossRef] [PubMed] [Google Scholar]
- Gonzales JP, Mishra GS. 1977. Polystomoides tunisiensis n. sp. (Monogenea, Polystomatidae) et Telorchis temimi n. sp. (Digenea, Telorchiidae). Deux nouvelles espèces de Trématodes de tortues paludines de Tunisie. Archives de l’Institut Pasteur de Tunis, 54, 29–38. [Google Scholar]
- Gray ME. 1993. Wetapolystoma almae n. gen., n. sp. (Monogenea: Polystomatidae) parasite of Bufo typhonius (Linnaeus, 1758) (Amphibia: Bufonidae) from Tropical Peru. Transactions of the Kansas Academy of Science, 98, 181–185. [CrossRef] [Google Scholar]
- Harris JR, Bergmire-Sweat D, Schlegel JH, Winpisinger KA, Klos RF, Perry C, Tauxe RV, Sotir MJ. 2009. Multistate outbreak of Salmonella infections associated with small turtle exposure, 2007–2008. Pediatrics, 124, 1388–1394. [CrossRef] [PubMed] [Google Scholar]
- Harris JR, Neil KP, Behravesh CB, Sotir MJ, Angulo FJ. 2010. Recent multistate outbreaks of human Salmonella infections acquired from turtles: A continuing public health challenge. Clinical Infectious Diseases, 50, 554–559. [CrossRef] [PubMed] [Google Scholar]
- Hatcher MJ, Dick JTA, Dunn AM. 2012. Disease emergence and invasions. Functional Ecology, 26, 1275–1287. [CrossRef] [PubMed] [Google Scholar]
- Héritier L, Valdeón A, Sadaoui A, Gendre T, Ficheux S, Bouamer S, Kechemir-Issad N, Du Preez L, Palacios C, Verneau O. 2017. Introduction and invasion of the red-eared slider and its parasites in freshwater ecosystems of southern Europe: risk assessment for the European pond turtle in wild environments. Biodiversity Conservation, 26, 1817–1843. [CrossRef] [Google Scholar]
- Héritier L, Verneau O, Smith KG, Coetzer C, Du Preez LH. Demonstrating the value and importance of combining DNA barcodes and discriminant morphological characters for polystome taxonomy (Platyhelminthes, Monogenea). Parasitology International, 2018. 67, 38–46. [CrossRef] [PubMed] [Google Scholar]
- Hidalgo-Vila J, Díaz-Paniagua C, Pérez-Santigosa N, de Frutos-Escobar C, Herrero-Herrero A. 2008. Salmonella in free-living exotic and native turtles and in pet exotic turtles from SW Spain. Research in Veterinary Science, 85, 449–452. [CrossRef] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755. [CrossRef] [PubMed] [Google Scholar]
- Jackson JA, Tinsley RC. 1998. Incompatibility of Protopolystoma xenopodis (Monogenea: Polystomatidae) with an octoploid Xenopus species from southern Rwanda. International Journal for Parasitology, 28, 1195–1199. [CrossRef] [PubMed] [Google Scholar]
- Jackson JA, Tinsley RC. 2001. Protopolystoma xenopodis (Monogenea) primary and secondary infections in Xenopus laevis. Parasitology, 123, 455–463. [CrossRef] [PubMed] [Google Scholar]
- Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM. 2009. Parasite spillback: A neglected concept in invasion ecology? Ecology, 90, 2047–2056. [CrossRef] [PubMed] [Google Scholar]
- Kok DJ, Du Preez LH. 1987. Polystoma australis (Monogenea): life-cycle studies in experimental and natural infections of normal and substitute hosts. Journal of Zoology, 212, 235–243. [CrossRef] [Google Scholar]
- Leidy J. 1888. Entozoa of the terrapin. Proceedings of the Academy of Natural Sciences of Philadelphia, 18, 127–128. [Google Scholar]
- Littlewood DTJ, Rohde K, Clough KA. 1997. Parasite speciation within or between host species? Phylogenetic evidence from site-specific polystome monogeneans. International Journal for Parasitology, 27, 1289–1297. [CrossRef] [PubMed] [Google Scholar]
- MacCallum GA. 1918. Studies on the Polystomatidae. Zoopathologica, 1, 103–120. [Google Scholar]
- Marin C, Ingresa-Capaccioni S, González-Bodi S, Marco-Jiménez F, Vega S. 2013. Free-living turtles are a reservoir for Salmonella but not for Campylobacter. PLoS One, 8, e72350. [CrossRef] [PubMed] [Google Scholar]
- Mermin J, Hutwagner L, Vugia D, Shallow S, Daily P, Bender J, Koehler J, Marcus R, Angulo FJ. 2004. Reptiles, amphibians, and human Salmonella infection: A population-based, case-control study. Clinical Infectious Diseases, 38, S253–261. [CrossRef] [PubMed] [Google Scholar]
- Meyer L, Du Preez L, Bonneau E, Héritier L, Quintana MF, Valdeón A, Sadaoui A, Kechemir-Issad N, Palacios C, Verneau O. 2015. Parasite host-switching from the invasive American red-eared slider, Trachemys scripta elegans, to the native Mediterranean pond turtle, Mauremys leprosa, in natural environments. Aquatic Invasions, 10, 79–91. [CrossRef] [Google Scholar]
- Paul AA. 1938. Life history studies of North American freshwater polystomes. Journal of Parasitology, 24, 489–510. [CrossRef] [Google Scholar]
- Pichelin S. 1995. The taxonomy and biology of the Polystomatidae (Monogenea) in Australian freshwater turtles (Chelidae, Pleurodira). Journal of Natural History, 29, 1345–1381. [CrossRef] [Google Scholar]
- Platt TR. 2000. Helminth parasite of the western painted turtle, Chrysemys picta belli (Gray), including Neopolystoma elizabethae n. sp. (Monogenea: Polystomatidae), a parasite of the conjunctival sac. Journal of Parasitology, 86, 815–818. [CrossRef] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics, 14, 817–818. [CrossRef] [PubMed] [Google Scholar]
- Poulin R, Paterson RA, Townsend CR, Tompkins DM, Kelly DW. 2011. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshwater Biology, 56, 676–688. [Google Scholar]
- Price EW. 1939. North American monogenetic trematodes. IV. The family Polystomatidae (Polystomatoidea). Proceedings of the Helminthological Society, 6, 80–92. [Google Scholar]
- Rudolphi CA. 1819. Entozoorum synopsis cui accedunt mantissa duplex et indices locupletissimi. Berolini: Sumibus A. Rücker, 811 p. [Google Scholar]
- Stunkard HW. 1916. On the anatomy and relationships of some North American trematodes. Journal of Parasitology, 3, 21–27. [CrossRef] [Google Scholar]
- Stunkard HW. 1924. On some trematodes from Florida turtles. Transactions of the American Microscopical Society, 43, 97–117. [CrossRef] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2013. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739. [Google Scholar]
- Tatem AJ, Rogers DJ, Hay SI. 2006. Global transport networks and infectious disease spread. Advances in Parasitology, 62, 293–343. [CrossRef] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680. [CrossRef] [PubMed] [Google Scholar]
- Tinsley RC, Jackson JA. 2002. Host factors limiting monogenean infections: a case study. International Journal for Parasitology, 32, 353–365. [CrossRef] [PubMed] [Google Scholar]
- Tinsley R, Stott L, York J, Everard A, Chapple S, Jackson J, Viney M, Tinsley MC. 2012. Acquired immunity protects against helminth infection in a natural host population: long-term field and laboratory evidence. International Journal for Parasitology, 42, 931–938. [CrossRef] [PubMed] [Google Scholar]
- Van Dijk PP, Iverson JB, Rhodin AGJ, Shaffer HB, Bour R. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. Chelonian Research Monographs, 5, 329–479. [Google Scholar]
- Verneau O, Palacios C, Platt T, Alday M, Billard E, Allienne J-F, Basso C, Du Preez LH. 2011. Invasive parasite threat: parasite phylogenetics reveals patterns and processes of host-switching between non-native and native captive freshwater turtles. Parasitology, 138, 1778–1792. [CrossRef] [PubMed] [Google Scholar]
- Warwick C, Lambiris AJL, Westwood D, Steedman C. 2001. Reptile-related salmonellosis. Journal of the Royal Society of Medicine, 94, 124–126. [CrossRef] [PubMed] [Google Scholar]
- Wilson AJ, Morgan ER, Booth M, Norman R, Perkins SE, Hauffe HC, Mideo N, Antonovics J, McCallum H, Fenton A. 2017. What is a vector? Philosophical Transactions B, 372, 2016085. [Google Scholar]
- Woodward DL, Khakhria R, Johnson WM. 1997. Human salmonellosis associated with exotic pets. Journal of Clinical Microbiology, 35, 2786–2790. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Verneau O, Quinn D, Smith KG, Malone JH & du Preez L. 2025. Role of Trachemys scripta elegans in polystome (Platyhelminthes, Monogenea, Polystomatidae) spillover and spillback following the trade of freshwater turtles in southern Europe and North America. Parasite 32, 30. https://doi.org/10.1051/parasite/2025022.
All Tables
Geographical areas of American and French aquatic environments investigated for turtles and their polystomes.
Polystome samples (worms and/or eggs) for which partial COI sequences were obtained in this study.
Polystome species and haplotypic diversity among American (Apalone ferox, A. spinifera, Chelydra serpentina, Chrysemys picta, Graptemys pseudogeographica, Kinosternon baurii, Pseudemys concinna, P. floridana, P. nelsoni, P. peninsularis and Trachemys scripta) and Mediterranean (Emys orbicularis and Mauremys leprosa) turtle species across natural North American and Mediterranean freshwater environments, as well as in outdoor turtle enclosures.
All Figures
 |
Figure 1 Schematic representation of the T. s. elegans range distribution according to Van Dijk et al. [50]. Original range in dark grey; range following human translocation in light grey. |
| In the text | |
 |
Figure 2 Radial tree depicting the phylogenetic relationships of the 134 discrete COI chelonian polystome haplotypes after Bayesian analysis. |
| In the text | |
 |
Figure 3 Phylogram of chelonian polystome species derived from the tree depicted in Figure 2 after collapsing haplotypes referring to the same species. Polystome species in blue refer to polystomes infecting the conjunctival sacs, in red the urinary bladder and in green the oral cavity of American turtles. Polystomatidae sp1 and sp2, which also infect American turtles, were not highlighted as the infection site was not determined. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


