| Issue |
Parasite
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 7 | |
| Number of page(s) | 20 | |
| DOI | https://doi.org/10.1051/parasite/2025001 | |
| Published online | 04 February 2025 | |
Review Article
Molecular pathogenesis of Cryptosporidium and advancements in therapeutic interventions
Pathogénèse moléculaire de Cryptosporidium et progrès dans les interventions thérapeutiques
1
College of Basic Medical Sciences, Shandong Second Medical University, Weifang, China
2
College of Pharmacy, Shandong Second Medical University, Weifang, China
3
College of Traditional Chinese Medicine, Shandong Second Medical University, Weifang, China
4
College of Clinical Medicine, Shandong Second Medical University, Weifang, China
* Corresponding author: jirui2012@126.com
Received:
7
August
2024
Accepted:
14
January
2025
Cryptosporidiosis, caused by a Cryptosporidium infection, is a serious gastrointestinal disease commonly leading to diarrhea in humans. This disease poses a particular threat to infants, young children, and those with weakened immune systems. The treatment of cryptosporidiosis is challenging due to the current lack of an effective treatment or vaccine. Ongoing research is focused on understanding the molecular pathogenesis of Cryptosporidium and developing pharmacological treatments. In this review, we examine the signaling pathways activated by Cryptosporidium infection within the host and their role in protecting host epithelial cells. Additionally, we also review the research progress of chemotherapeutic targets against cryptosporidia-specific enzymes and anti-Cryptosporidium drugs (including Chinese and Western medicinal drugs), aiming at the development of more effective treatments for cryptosporidiosis.
Résumé
La cryptosporidiose, causée par une infection à Cryptosporidium, est une maladie gastro-intestinale grave entraînant généralement une diarrhée chez l’homme. Cette maladie constitue une menace particulière pour les nourrissons, les jeunes enfants et les personnes dont le système immunitaire est affaibli. Le traitement de la cryptosporidiose est difficile en raison de l’absence actuelle de traitement ou de vaccin efficace. Les recherches en cours se concentrent sur la compréhension de la pathogénèse moléculaire de Cryptosporidium et le développement de traitements pharmacologiques. Dans cette revue, nous examinons les voies de signalisation activées par l’infection à Cryptosporidium au sein de l’hôte et leur rôle dans la protection des cellules épithéliales de l’hôte. De plus, nous passons également en revue les progrès de la recherche sur les cibles chimiothérapeutiques contre les enzymes spécifiques de Cryptosporidium et les médicaments anti-Cryptosporidium (y compris les médicaments chinois et occidentaux), en visant le développement de traitements plus efficaces contre la cryptosporidiose.
Key words: Cryptosporidium / Cryptosporidiosis / Signaling pathways / Enzymatic targets / Drug therapy
Edited by: Jean-Loup Justine
© Y. Lu et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cryptosporidium, one of the most widely distributed intestinal parasites globally [166], was first defined as a human pathogen in 1976 [145]. Human infections with Cryptosporidium are predominantly caused by C. hominis and C. parvum [62]. Belonging to the Coccidia class within the phylum Apicomplexa, Cryptosporidium possesses distinct features that distinguish it from other members of the Coccidia group [83]. Its intracellular and extracytoplasmic localization is a key characteristic, along with the formation of unique “feeding” structures. In addition, Cryptosporidium produces two morphologically distinct types of oocysts, i.e., thick-walled and thin-walled, which differ markedly from the oocysts of other Coccidia by lacking structures like sporocysts or micropyles. Despite the common use of chlorine as a disinfectant in water to eliminate numerous microorganisms, Cryptosporidium oocysts exhibit resistance to chlorine and various environmental stresses, such as extreme temperature changes [77]. This resilience enables the oocysts to remain infectious outside of a host for long periods.
Cryptosporidium infection can lead to damage to intestinal epithelial cells in both humans and other mammals. This damage disrupts the tight junctions of the intestinal epithelium, affecting intestinal barrier function. It also hinders the development of villi and reduces the absorptive capacity of the intestinal surface, leading to malnutrition, dehydration, and diarrhea [105]. The diarrheal symptoms associated with cryptosporidiosis pose a major threat to human health [103]. Malnourished children are particularly susceptible to Cryptosporidium infection, which can lead to recurrent or persistent infections and even death [40, 108, 127]. In adults, cryptosporidiosis commonly occurs in immunocompromised individuals, especially people with AIDS and organ transplant recipients [49].
Cryptosporidium infection is a widespread global disease, with a global pooled prevalence of 7.6% [51]. The World Health Organization (WHO) classified the disease as one of the neglected diseases in 2004 [162] and identified it as one of the six major diarrheal diseases [196]. Cryptosporidiosis is more commonly found in developing countries compared to developed nations [17]. It causes more than 50 million episodes of diarrhea and 48,000 deaths annually among children under 5 years of age in developing countries, especially in sub-Saharan Africa [66, 101]. The infection is also prevalent among immunocompromised populations in low- and middle-income countries, notably among HIV-positive individuals [9, 121, 139, 172, 178]. In Africa, the prevalence of cryptosporidiosis among people with HIV ranges from 5.6% to 25.7%; in Asia, from 3.7% to 45.0%; in South America, from 5.6% and 41.6%; and in Europe, from 2.6% to 15.1% [178].
Cryptosporidium infection is mainly transmitted through the fecal-oral route and can occur due to contact with animals, feces, or contaminated water or food [160]. Respiratory secretions and aerosolised droplets from coughing can also serve as means of transmissions, leading to pulmonary infections [67]. The pathogen poses a significant threat to the water industry [38], with nearly 35% of reported cryptosporidiosis outbreaks in the United States between 2009 and 2017 linked to exposure to treated recreational water sources, such as swimming pools and water playgrounds. This exposure was associated with nearly 57% of the reported cases during this period [68]. A recent report from Kenya indicated an outbreak of diarrhea caused by Cryptosporidium hominis infection likely stemming from contact with contaminated waters during swimming [170]. Contact with infected cattle and people in childcare settings can also spark outbreaks [68]. Additionally, Cryptosporidium is recognized as an important foodborne pathogen, contributing to over 40 recorded foodborne outbreaks and more than 8 million cases of foodborne illness annually [200]. These factors are considered high-risk factors for Cryptosporidium infection (Table 1).
Risk factors for Cryptosporidium infection.
The objective of this review was to comprehensively analyze the molecular mechanisms underlying Cryptosporidium infection. This includes elucidating the signaling pathways activated by Cryptosporidium and their role in host defense mechanisms against the infection. Furthermore, we aimed to discuss potential chemotherapeutic targets and recent advancements in drug development for Cryptosporidium. Uncovering the unique metabolic pathways and survival strategies of Cryptosporidium has led to the identification of targets for specific enzymes, resulting in significant strides in drug development against this parasite. In addition, we offer an overview of existing research on drugs targeting Cryptosporidium infections, ranging from Chinese herbal remedies to clinical therapeutics. Through this review, we aspire to provide scientific insight and theoretical support to facilitate the development of more effective treatments and medications for cryptosporidiosis.
Molecular pathogenesis of Cryptosporidium
To date, more than 200 drugs have been screened for cryptosporidiosis, but an optimal therapeutic intervention remains elusive. While several vaccine candidates have demonstrated limited protective effects through targeting specific genes [40], their efficacy falls short of therapeutic requirements. This situation fully demonstrates the urgent need for new therapeutic strategies to address this pressing public health challenge. To overcome this challenge, future research on Cryptosporidium should focus more on its molecular mechanisms, especially the signaling pathways implicated in the process of Cryptosporidium infection. Understanding how these signaling pathways are regulated and how they contribute to the activation of host defense mechanisms is crucial for the control and prevention of Cryptosporidium infection. Delving deeper into this field will establish a solid theoretical basis for the creation of innovative therapeutic strategies.
The NF-κB pathway plays a role in regulating host epithelial cell RNA during Cryptosporidium infection
Activation of the NF-κB pathway is a critical response of epithelial cells to pathogen infections. NF-κB is an important group of nuclear transcription factors consisting of five members, namely p50, p52, p65 (Rel A), c-Rel, and Rel B [69]. In its inactivated state, NF-κB is bound to the inhibitory factor IκB and remains inactive in the cytoplasm [78]. Various stimuli, such as bacterial, viral, and protozoan parasitic infections, can trigger the activation of NF-κB [37]. Upon activation, NF-κB translocates to the nucleus, where the transcription factors specifically bind to the κB site in the promoter region of target genes. This binding initiates gene transcription, regulating the expression of a variety of host genes [211, 212].
Host infection with Cryptosporidium activates the NF-κB signaling pathway. Ongoing research is uncovering the mechanisms through which NF-κB signaling regulates the role of epithelial cell RNA in defense against Cryptosporidium infection. This includes the regulation of mRNA, miRNA, lncRNA, and circRNA (Fig. 1). A recent study has identified a new role for NF-kB signaling in controlling CX3CL1 expression during Cryptosporidium parvum infection. The expression of CX3CL1 is increased in biliary epithelial cells during Cryptosporidium parvum infection, and this induction may be associated with the downregulation of miR-424 and miR-503. The downregulation of miR-424 and miR-503 is synergistically regulated by the NF-kB signaling pathway, leading to the increased expression of CX3CL1. The release of CX3CL1 contributes to the enhancement of defense against Cryptosporidium parvum by attracting immune cells to the mucosa [210].
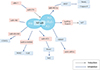 |
Figure 1 Schematic diagram of the NF-κB pathway involved in the regulation of host epithelial cell RNA during Cryptosporidium infection. |
The NF-κB signaling pathway is involved in the regulation of circRNAs in Cryptosporidium-infected host cells, offering a new perspective on the regulatory mechanisms of Cryptosporidium infection. In a study, it was observed that ciRS-7 was significantly upregulated in HCT-8 cells after Cryptosporidium parvum infection. RelA, a subunit of the NF-κB signaling pathway, plays a role in inhibiting apoptosis in epithelial cells, which can benefit parasite reproduction [41]. RelA is a direct target of miR1270, and ciRS-7 has the ability to affect the NF-κB pathway, promoting Cryptosporidium parvum reproduction by modulating the miR-1270/RelA axis [195].
Elevated levels of TLR4 and NF-κB have been observed in Cryptosporidium parvum-infected cholangiocytes, confirming the activation of the TLR/NF-κB signaling pathway [42]. Studies have revealed that the TLR2/TLR4-NF-κB signaling pathway is involved in the expression of miR-942-5p [179, 208]. Changes in miR-942-5p expression after Cryptosporidium parvum infection play a key role in the immune response of host epithelial cells. Activation of this pathway by Cryptosporidium parvum leads to the upregulation of miR-942-5p, and inhibition of TLR2 and TLR4 results in the significant downregulation of miR-942-5p. Additionally, miR-942-5p, which targets the IFI27 gene, has been shown to attenuate apoptosis in HCT-8 cells early in Cryptosporidium parvum infection, influencing the burden of Cryptosporidium parvum infection [188]. Additionally, Cryptosporidium parvum can mediate the expression of miR-181d in the TLR2/TLR4-NF-κB signaling pathway via the p50 subunit [61]. miR-181d expression is initially reduced during infection, while TLR2, TLR4, NF-κB, and myD88 in the pathway are upregulated. After inhibition of NF-κB and p50 subunit, miR-181d expression is increased. Recent findings suggest that the TLR/NF-κB signaling pathway contributes to the upregulation of miR-4521 expression during Cryptosporidium parvum infection. miR-4521 regulates BCL2-mediated apoptosis and targets FOXM1 to enhance Cryptosporidium parvum reproduction in HCT-8 cells [194]. This study provides a new theoretical framework for understanding the involvement of the NF-κB signaling pathway in the role of miRNA in Cryptosporidium infection.
In a study by Nicholas W. Mathy et al., it was found that Cryptosporidium parvum infection of the host intestinal epithelium leads to a significant increase in the expression of lncRNA Nostrill [135]. The study demonstrated that inhibiting the upregulation of Nostrill and the downregulation of the NF-kB response gene Cxcl2 using an NF-kB inhibitor was effective, highlighting the crucial role of NF-kB signaling. Furthermore, Nostrill was shown to promote the recruitment of NF-kB p65 to the Irf7 gene promoter, thereby promoting Irf7 expression and strengthening the defense of the small intestinal epithelium against Cryptosporidium parvum [135]. Another report examining lncRNA expression in intestinal epithelial cells after Cryptosporidium parvum infection revealed that lncRNA NR_045064, which is controlled by NF-κB signaling, was significantly upregulated. Functionally, the induction of NR_045064 was found to enhance the transcription of defense genes and inhibit Cryptosporidium parvum infection [114]. These findings suggest that the NF-κB pathway plays a role in regulating lncRNA-mediated defense in intestinal epithelial cells against Cryptosporidium infection, indicating a promising avenue for future research in anti-infection strategies.
The TLR/MyD88/NF-кB signaling pathway in intestinal epithelial cells has been identified as a critical component of defense against Cryptosporidium parvum infection [30, 41]. When Cryptosporidium parvum infects the intestinal epithelium, it triggers the activation of the NF-кB signaling pathway, leading to the downregulation of Alkbh5, which is responsible for elevated levels of mRNA m6A methylation [186]. m6A methylation plays an important role in the regulation of the immune response [74] and enhances the defense against Cryptosporidium parvum in epithelial cells. MyD88, a key gene upstream of the NF-кB pathway, is involved in this process, and the levels of Alkbh5 expression were not seen in MyD88 knockdown intestinal epithelial cells. Chromatin immunoprecipitation (ChIP) analysis revealed a high occupancy of the Alkbh5 gene by the NF-кB subunit p65, indicating that Alkbh5 induces m6A methylation under the influence of the activated NF-кB pathway [186].
Understanding whether the regulation involved in Cryptosporidium-induced NF-kB signaling benefits the host, the parasite, or both is crucial. Clarifying these dynamics could help to develop more effective strategies to combat Cryptosporidium infection.
The PI3K/Akt signaling pathway is involved in autophagy regulation during Cryptosporidium infection
EGFR is a tyrosine kinase receptor located in the cell membrane that plays a role in the activation of various signaling systems, primarily through the MAPK pathway and the PI3K/Akt pathway [181]. Upon stimulation, cellular EGFR becomes phosphorylated and triggers the PI3K/Akt signaling pathway, which is essential for regulating basic cellular functions [84]. In the context of Cryptosporidium parvum, the parasite has been found to activate the EGFR-PI3K/Akt pathway to manipulate host cell autophagy [193]. Autophagy is a cellular mechanism used for defense against invading pathogens [23], but parasites can evade the host immune response by manipulating this process [156]. Studies have shown that when HCT-8 cells are infected with Cryptosporidium parvum for 2–8 h, there is an elevated expression of EGFR-P and Akt-P (phosphorylated forms of EGFR and Akt, respectively). Inhibition of EGFR results in a significant reduction in the phosphorylation levels of EGFR and Akt. Akt, a downstream effector of PI3K, is activated through PI3K-mediated phosphorylation [168]. Inhibition of PI3K leads to a reduction in Akt expression. Additionally, LC3 serves as a crucial marker in the autophagic process [131]. When EGFR and Akt are blocked, upregulation of LC3 expression enhances autophagy and inhibits parasite proliferation [193]. This suggests that Cryptosporidium parvum can counteract autophagy by manipulating the EGFR-PI3K/Akt pathway to maintain its survival within the host. Research has also shown that Cryptosporidium parvum induces cellular autophagy through the PI3K/Akt/mTOR signaling pathway. Transmission electron microscopy has revealed the presence of autophagosomes in Cryptosporidium parvum-infected cells, and a decrease in the phosphorylation levels of PI3K, Akt, and mTOR proteins was observed after 8 h. The use of rapamycin (Rapa), an mTOR inhibitor, was able to inhibit mTOR signaling [3]. In cells pretreated with Rapa, the proliferation of Cryptosporidium parvum was significantly inhibited at 12 h post-infection. This indicates that Cryptosporidium parvum induces autophagy via the PI3K/Akt/mTOR pathway in HCT-8 cells, and cellular autophagy inhibits the early development of Cryptosporidium parvum [116]. While research on the interaction between Cryptosporidium and the PI3K/Akt pathway is in its infancy, further expansion in this area may reveal novel therapeutic and preventive strategies for combatting Cryptosporidium infections.
The TLR4/STAT1 signaling pathway is involved in Cryptosporidium infection
Toll-like receptors (TLRs) play a key role in immune activation and pathogen recognition [10, 24], with elevated expression of TLR4 observed during Cryptosporidium parvum infection [189]. Recent studies, including our own research, have revealed an upregulation of TLR4 expression in a mouse model of Cryptosporidium parvum infection [97]. The TLR4-mediated responses are essential for the clearance of Cryptosporidium parvum bile duct infections in vivo. The absence of this pattern recognition receptor can lead to altered inflammatory responses and worsen hepatobiliary pathology [146]. STAT1 serves as a key regulator of IFN-γ signaling. During Cryptosporidium parvum infection, the parasite inhibits STAT1 activity [44, 154]. Additionally, the expression of interferon regulatory factor-1 (IRF-1) is reduced post-infection, impacting the regulation of various innate and adaptive immune responses. At the cellular level, IFN-γ can bind to its receptor (IFN-γR) to initiate signaling cascades and activate STAT1 [180]. A recent study has shown that Chitosan can target the TLR4/STAT1 pathway. By downregulating TLR4 signaling and upregulating the STAT1 signaling pathway to induce IFN-γ signaling, Chitosan can attenuate Cryptosporidium parvum infection [151]. This research sheds light on potential therapeutic strategies that target the TLR4/STAT1 pathway to combat Cryptosporidium infections effectively.
Enzymatic targets for drug interventions against Cryptosporidium infection
Current therapeutic approaches for cryptosporidiosis are predominantly limited to pharmacological interventions. Nitazoxanide (NTZ), while approved by the FDA for treating human cryptosporidiosis, shows limited efficacy in immunocompromised and HIV-infected patients, necessitating the development of new therapeutic strategies. Cryptosporidium-specific enzymes, which play key roles in the parasite’s unique metabolic pathways and survival mechanisms, represent promising therapeutic targets. Our research focuses on these enzymatic targets (Table 2) with the aim of developing more effective treatments. However, the presence of homologous enzymes in mammalian cells presents a significant challenge, as inhibitors targeting these enzymes affect both parasite and host cellular functions, potentially triggering adverse effects. Therefore, drug development efforts must prioritize strategies that selectively target parasite-specific enzymes. To advance this objective, we will evaluate potential drugs against these targets through in vitro and in vivo experiments to provide a scientific basis for the development of novel therapeutic drugs for cryptosporidiosis.
Enzyme targets and specific compounds targeted towards Cryptosporidium.
Lactate dehydrogenases (LDH)
The glycolytic pathway plays a key role in ATP production, with the lactate dehydrogenase (LDH) enzyme being pivotal in catalyzing the conversion of pyruvate to lactate and the oxidation of nicotinamide adenine dinucleotide (NADH to NAD+). The regenerative cycle of NAD+ within glycolysis is essential for generating ATP, a vital energy source for cell survival and development [63, 94, 134]. Parasitic mitochondria, including those of Cryptosporidium parvum, lack functional oxidative phosphorylation and a complete tricarboxylic acid (TCA) cycle. Consequently, these parasites rely on anaerobic respiration for their energy needs, with LDH playing a crucial role in meeting these requirements [100]. Thus, inhibiting LDH activity may potentially result in the death of Cryptosporidium. Lactate dehydrogenase inhibitors such as gossypol and FX11 demonstrate inhibitory activity against Cryptosporidium parvum lactate dehydrogenase (CpLDH), albeit without specificity [201]. Thus, inhibitors targeting CpLDH may also affect LDH activity in mammalian cells. While CpLDH shares structural similarities with human LDH, detailed structural analysis reveals distinct differences in both the active site and cofactor binding regions. These variations result in CpLDH exhibiting lower affinity for NAD+/NADH and may affect inhibitor selectivity [45]. Studies have identified NSC158011 and NSC10477 as selective CpLDH inhibitors that effectively suppress the growth and replication of Cryptosporidium parvum both in vitro and in vivo, demonstrating inhibitory effects at concentrations below the threshold of mammalian cell toxicity [115]. Given these findings, a comprehensive evaluation of this inhibitor’s potential effects on humans remains crucial in the development of CpLDH-targeted inhibitors.
Calcium-dependent protein kinas 1 (CDPK1)
Similar to other eukaryotic cells, protein kinases play a crucial role in the biology of protozoan parasites such as Cryptosporidium [140], making them potential targets for anti-cryptosporidial drug development. Apicomplexan CDPKs are essential enzymes involved in parasite gliding motility, invasion, and exit [173]. However, the catalytic sites of these CDPKs contain a glycine instead of the bulkier gatekeeper residues typically found in mammalian CDPKs. Bumped kinase inhibitors (BKI) target parasitic CDPKs, and a series of inhibitor compounds have been developed [35]. Previous reports have shown that BKI-1517 and BKI-1553 exhibit anti-cryptosporidial activity in both in vivo and in vitro assays [34, 163]. Further investigations by Hulverson et al. on BKI-1294, BKI-1369, BKI-1534, and BKI-1649 revealed that all these compounds reduce Cryptosporidium parvum infections [91]. BKI-1294 reduces oocyst shedding but does not effectively address diarrhea and dehydration in calves [113]. BKI-1369 shows promising anti-cryptosporidiosis effects in the neonatal calf and piglet models infected with Cryptosporidium parvum [89, 112], positioning it as a potential candidate for disease treatment in animal models. Although these BKIs demonstrate potent inhibitory activity against the human ether-à-go-go-Related Gene (hERG), which is associated with human QT syndrome and cardiotoxicity, caution is advised in their use for human cryptosporidiosis treatment [173]. In addition, other BKI analogues structured with 5-aminopyrazole-4-carboxamide and pyrrolopyrimidines, have shown effectiveness in IFN-γ KO mouse models of C. parvum infection. However, these compounds exhibit certain pharmacological properties that preclude their immediate application in human treatments [88, 90]. Nevertheless, inhibitors targeting CpCDPK1 present compelling evidence for their therapeutic potential, establishing a promising direction for clinical research in cryptosporidiosis treatment.
Plasmodium lipid kinase (PI(4)K)
Phosphatidylinositol-4-oh kinase (PI(4)K) is an enzyme found in the apicomplexan protozoa Plasmodium, where phosphorylated lipid molecules play crucial roles in intracellular signaling and transport [137]. Researchers have targeted the Cryptosporidium lipid kinase PI(4)K and, through screening efforts, have optimized a potential inhibitory compound known as the pyrazolopyridine derivative KDU731. KDU731 exhibits selective inhibition of this enzyme, effectively reducing oocyst shedding, diarrhea duration, and dehydration symptoms in experimentally infected calves, with no significant adverse effects observed [130]. Similarly, EDI048, another selective Cryptosporidium PI(4)K inhibitor, shows potent antiparasitic activity and demonstrates therapeutic efficacy when administered orally in animal models, successfully reducing oocyst shedding and ameliorating diarrhea symptoms [129]. Previous studies have established the effectiveness of both compounds in animal models, suggesting that successful progression through clinical trials could address the current therapeutic void in cryptosporidiosis treatment.
Nucleoside diphosphate kinase (NDK)
Cryptosporidium nucleoside diphosphate kinase (NDK) is essential for the biosynthesis of nucleoside triphosphate and normal metabolism in Cryptosporidium, which can be used as a strategic target for therapeutic development. In vitro studies have demonstrated that NDK suppression significantly inhibited Cryptosporidium proliferation [32]. Additionally, the anti-Cryptosporidium activity of the NDK inhibitor ellagic acid (EA) was tested, with EA demonstrating a reduction in Cryptosporidium loads (EC50 = 15–30 μM) without showing human cytotoxicity [32]. While NDK is conserved across mammalian cells, preliminary observations demonstrate that EA exhibits substantially higher inhibitory activity against semi-purified Cryptosporidium NDK compared to its human homolog [32], establishing a basis for the development of selective Cryptosporidium NDK inhibitors. Further research by Castellanos-Gonzalez et al. introduced a single-stranded RNA (ssRNA)/Argonaute (Ago) complex to silence Cryptosporidium parvum NDK in both in vivo and ex vivo models. The study showed that ssRNA/Ago attenuates in vitro Cryptosporidium parvum infections and reduces Cryptosporidium parvum burden in infected mice, suggesting its potential as a treatment for cryptosporidiosis [33].
Inosine monophosphate dehydrogenase (IMPDH)
Inosine monophosphate dehydrogenase (IMPDH) is an enzyme essential for catalyzing the conversion of inosine monophosphate (IMP) to xanthine monophosphate (XMP), a pivotal and rate-limiting step in guanine nucleotide biosynthesis [81]. Guanine nucleotides serve as precursors for glycosylation, RNA, DNA and tetrahydrobiopterin synthesis across various organisms [13, 14].Considering the significance of guanine nucleotides, inhibition of Cryptosporidium IMPDH represents a promising therapeutic strategy for cryptosporidiosis. The distinctive evolutionary origin of Cryptosporidium IMPDH, which is structurally divergent from its host counterpart, provides a molecular foundation for developing selective inhibitors that can effectively target the parasitic enzyme, while minimizing interference with host cellular functions [104]. P131, an inhibitor of Cryptosporidium IMPDH, has displayed potent anti-cryptosporidial activity in mice [70] and exhibits selective binding to the NAD site within Cryptosporidium IMPDH [147]. Moreover, a peptide-based inhibitor known as phylomer has shown efficacy in targeting Cryptosporidium IMPDH, with phylomers 8 and 24 demonstrating the inhibition of Cryptosporidium parvum proliferation in HCT-8 cells [96]. In a study by Sarwono et al., three irreversible IMPDH inhibitors (disulfiram, bronopol, and ebselen) were screened. Oral administration of disulfiram and bronopol in a severe combined immunodeficiency (SCID) mouse model of cryptosporidiosis was found to reduce oocyst excretion [161]. A follow-up study suggested the presence of a potentially novel enzyme in the parasite that might utilize a unique mechanism to synthesize XMP, potentially circumventing the need for IMPDH [149]. This discovery indicates that drug development targeting Cryptosporidium IMPDH must address dual considerations, namely the specificity relative to host IMPDH and the possibility of metabolic pathways that could circumvent IMPDH inhibition.
Thymidylate-synthetase dihydrofolate reductase (TS-DHFR)
Studies on Cryptosporidium nucleotide metabolism have identified a variety of proteins crucial for the parasite’s life cycle. Among these, thymidylate synthase-dihydrofolate reductase (TS-DHFR), a bifunctional enzyme involved in the synthesis of monophosphate thymine and folate in Cryptosporidium, stands out. The enzyme exists as a homodimer, with each monomer containing a unique swap domain known as a “crossover helix”, which is not present in human DHFR [132]. This structural divergence makes TS-DHFR an attractive target for selective inhibitor development [71, 133, 199]. Modified pyrimidine derivatives have been evaluated as inhibitors of Cryptosporidium hominis (Ch) TS-DHFR, demonstrating an EC50 of 1–5 μM in cell cultures. These inhibitors significantly impeded parasite proliferation [110]. Additionally, Ruiz et al. used virtual screening and structure-guided modeling to identify compounds targeting ChTS-DHFR, leading to the discovery of 15D17 as the first covalent inhibitor designed to target the non-catalytic pocket of ChTS-DHFR [159]. These studies emphasize the development of selective inhibitors targeting Cryptosporidium-specific enzymes, while minimizing interactions with host cell homologs, establishing novel therapeutic approaches for cryptosporidiosis treatment.
tRNA synthetases
A tRNA synthetase is responsible for binding specific amino acid residues to selected tRNAs, a fundamental step in protein synthesis. Inhibitors targeting tRNA synthetases have been explored in the development of anti-cryptosporidial drugs, such as those focused on prolyl-tRNA synthetase [95] and leucyl-tRNA synthetase [148]. Vinayak et al. found that BRD7929, a bicyclic azopyridine compound targeting Cryptosporidium phenylalanyl-tRNA synthetase, showed significant anti-cryptosporidial effects in both in vitro and in vivo experiments [177]. Another research study revealed that compound 2093, a Cryptosporidium methionyl-tRNA synthetase inhibitor, displayed potent antiparasitic activity against Cryptosporidium, while concurrently inhibiting human mitochondrial methionyl-tRNA synthetase. Although short-term treatment may be tolerated, further investigation is warranted to fully evaluate its therapeutic potential and safety profile [29]. Despite the promising results, challenges such as recurrent infections and emerging resistance have been observed, particularly in Cryptosporidium parvum-infected calves treated with compound 2093 [75]. Therefore, the development of new treatment modalities should be accompanied by a focus on combating resistance. Studies have reported that compound 5, a cladosporin derivative, is a potent inhibitor of Cryptosporidium Lysyl tRNA synthetase (KRS), and also acts against human KRS (HsKRS), but with stronger activity against Cryptosporidium parvum (Cp) KRS [22]. While the compound significantly reduced oocyst shedding in murine models, administration at higher doses (50 mg/kg orally) induced toxicity, probably due to inhibition of mammalian KRS, resulting in disruption of protein synthesis, cell metabolism, and physiological functions, with potential implications for organ dysfunction. Although tRNA synthetase represents a potential target for anticryptosporidial therapeutics, the development of inhibitors must carefully address selective targeting of parasitic enzymes, as insufficient specificity may result in host enzyme inhibition, leading to adverse effects and potential health complications.
Subtilisin-like serine protease 1 (SUB1)
Egress, the process by which motile merozoites are released from infected cells, is a key step in the Cryptosporidium life cycle, facilitating the spread of infection within the host epithelium. Research in Plasmodium falciparum has highlighted the role of Subtilisin-like serine protease 1 (SUB1) in cleaving parasite vesicle membranes (PVM) and host cytosolic membranes, promoting infection spread [8, 25]. Additionally, the Plasmodium falciparum SUB1 inhibitory compound, peptide boronic acid, has shown the ability to inhibit parasite replication in vitro [117]. Drawing parallels from plasmodium research, it was hypothesized that Cryptosporidium SUB1 may play a similar role in egress, making it a potential target for inhibiting Cryptosporidium infection. Reports have demonstrated that blocking SUB1 significantly inhibits Cryptosporidium parvum infection in cell culture [60]. A recent study has specifically identified SUB1 as a key mediator of Cryptosporidium parvum egress from host cells [142]. Nava et al. conducted Cryptosporidium parvum SUB1 mRNA silencing (ΔSUB1), leading to a 95% reduction in schizonts released in vitro compared to wild-type strains. Additionally, a higher number of intracellular parasites were observed in ΔSUB1 cells than in the wild-type control, indicating that SUB1 silencing effectively blocked Cryptosporidium egress and infection spread [142]. These findings suggest that targeting Cryptosporidium SUB1 could be a significant avenue for future vaccine and drug development efforts to combat Cryptosporidium infection and transmission.
Cysteine protease (cryptopains)
In Cryptosporidium parvum, 20 genes encoding clan CA (papain-like) cysteine proteases (CPs) have been reported, of which 5 genes encode “Cryptopains”, including 3 cathepsin L-like members and 2 cathepsin B-like members [6]. However, only cryptopain-1 has been biochemically analyzed and was found to be actively transcribed and expressed in sporozoites (the infective stage of the parasite) [141]. A cysteine protease inhibiting compound, N-methyl piperazine-Phe-homoPhe-vinylsulfone phenyl (K11777), has been identified as orally bioavailable with a tolerable safety and toxicity profile [2]. K11777 has been shown to inhibit Cryptosporidium parvum growth in a concentration-dependent manner in a variety of mammalian cell lines, including MDCK, HCT8, I407, and CaCo-2 [143]. The compound displays favorable selectivity, with minimal cytotoxicity observed in MDCK cells at concentrations up to 100 μM and negligible effects on human cells. In vivo efficacy studies revealed that K11777 effectively treats cryptosporidial infections in murine models, significantly improving infection parameters and preventing disease recurrence.
Long-chain fatty acyl-coenzyme A synthetase (LC-ACS)
Long-chain fatty acid acyl-coenzyme A synthetase (LC-ACS), a key enzyme in fatty acid metabolism, represents a potential novel therapeutic target for cryptosporidiosis intervention. Adenosine monophosphate (AMP) binds to long-chain (LC) fatty acyl coenzyme A synthetase (ACSs), facilitating the thioesterification reaction that converts free fatty acids and coenzyme A into fatty acyl coenzyme A [16]. ACS not only catalyzes this important reaction, but also serves as a fatty acid transporter, enabling the activation of acyl coenzyme A thioesters from fatty acids, which are crucial intermediates in various pathways [182, 213]. In mammalian systems, different ACS isoforms regulate tissue-specific fatty acid metabolism and storage in hepatic, adipose, and muscular tissues. Cryptosporidium parvum genome encodes three distinct LC-ACS isoforms, each serving stage-specific functions during parasite development [73]. Research conducted by Guo et al. demonstrated that Triacsin C effectively inhibits Cryptosporidium parvum ACS activity. The compound exhibits significant antiparasitic effects in vitro and greatly reduces oocyst burden in IL-12 knockout mouse models without apparent toxicity [72]. While Triacsin C inhibits LC-ACS activity both in humans and animals, its selective impact on lipid metabolism pathways, specifically preserving phospholipid fatty acid recycling, provides a mechanistic explanation for its favorable toxicity profile in animal studies. Recent studies have identified R134 as a potential candidate for targeting Cryptosporidium parvum LC-ACS enzyme isoforms with improved efficacy and pharmacokinetic parameters [39]; however, further experimental validation is required to definitively establish its anticryptosporidial activity.
Research on Chinese and Western medicinal drugs against Cryptosporidium infection
Controlling cryptosporidiosis poses a significant challenge in both veterinary and human medicine. Nitazoxanide, approved by the US FDA for the treatment of cryptosporidiosis [103], is effective primarily in immunocompetent patients [7]. In our comprehensive review, we analyzed previously published studies on drugs for combating Cryptosporidium infection, including Chinese herbal remedies and clinical therapeutics (Tables 3 and 4). Our investigation spans a wide range of experiments, including those conducted in animal models and human clinical trials. The evaluation criteria considered in the experiments comprise clinical improvement (such as reduction in diarrhea, changes in body weight, and enhancement of overall host health) and parasite clearance (involving the clearance of host fecal oocysts). These assessments are crucial in determining the efficacy of the treatment against cryptosporidiosis.
Anti-Cryptosporidium efficacy of different western medications in human clinical and host models.
Anti-Cryptosporidium effects of various traditional Chinese medicines in farm animals and host models.
Drugs in use for clinical treatment
Nitazoxanide
Currently, nitazoxanide (NTZ) stands as the only FDA-approved drug specifically designed for the treatment of cryptosporidiosis. NTZ is a thiazole antiparasitic drug known for its broad spectrum of antiparasitic activity [26, 209]. The drug primarily disrupts the function of pyruvate:ferredoxin oxidoreductase (PFOR) [165]. NTZ acts as an inhibitor of PFOR, disrupting anaerobic energy transfer reactions and inhibiting parasite growth [87]. The precise mechanism by which NTZ hampers Cryptosporidium infection remains incompletely understood, as these parasites possess a unique PFOR with a fused c-terminal cytochrome P450 structural domain [158]. Several studies have delved into NTZ clinical trials targeting cryptosporidiosis, yielding significant results [52, 157]. However, in immunocompromised individuals, the duration of diarrhea is prolonged, and parasite clearance is less effective [1, 15]. Nevertheless, significant alleviation of diarrheal symptoms has been reported in HIV-seronegative children treated with NTZ [15]. Improving the effectiveness of medications is a crucial focus. Research has demonstrated that octaarginine (R8) favors the uptake of NTZ. Treatment of Cryptosporidium parvum-infected human ileocecal carcinoma cells (HCT-8) with NTZ-R8 has shown enhanced inhibition of Cryptosporidium parvum growth compared to NTZ alone [144]. To further explore the anti-Cryptosporidium effects of NTZ, animal experiments were conducted using selected appropriate animal models and standard experimental methods to validate the efficacy of NTZ. Treatment of Cryptosporidium sp.-infected goats with NTZ has proven to effectively reduce oocyst shedding, demonstrating a therapeutic effect [176]. This efficacy was similarly observed in a mouse model of Cryptosporidium spp. infection [21, 53, 190]. Therapeutic benefits can be achieved through the administration of medications alone, but combinations of medications may enhance their effectiveness. Studies have shown that the combination of secnidazole (SEC) with NTZ [126], as well as the combined use of low-dose clofazimine (CFZ) and half-dose NTZ [58], resulted in significant reductions in disease compared to NTZ monotherapy. Due to the diverse mechanisms of action exhibited by different drugs, combining them can amplify their efficacy, while reducing the risk of adverse effects associated with a single drug. In addition, drug combinations can elevate the success rate of treatment, shorten the treatment period, and enhance overall treatment outcomes, providing substantial benefits for managing cryptosporidiosis.
Clofazimine
Clofazimine (CFZ) is a drug known for its anti-inflammatory properties [198] and has traditionally been used in the treatment of leprosy [43]. Recent studies have explored the potential of CFZ in the treatment of cryptosporidiosis. Experiments conducted with CFZ in a mouse model of Cryptosporidium parvum infection have shown promise in ameliorating symptoms associated with cryptosporidiosis [58, 123]. However, Zhang et al. reported no significant therapeutic effect of CFZ in a phase 2a clinical trial involving HIV-infected individuals with cryptosporidiosis [203]. This lack of efficacy may be due to the dose or concentration of CFZ not reaching therapeutic levels. Additionally, CFZ has not been found to be effective in treating cryptosporidiosis in immunocompromised HIV populations [93]. To enhance the effectiveness of CFZ in cryptosporidiosis treatment, the dosage of CFZ needs to be increased. As CFZ is insoluble, poorly absorbed and utilized, its oral bioavailability can be improved through SNEDDS (Self-NanoEmulsifying Drug Delivery System) formulations [191]. Furthermore, using CFZ nanoparticle formulations to treat cryptosporidiosis may be another viable approach. These strategies offer promising avenues for the future utilization of CFZ in cryptosporidiosis treatment.
Paromomycin
Paromomycin, an antibiotic classified under the aminoglycoside group, has been studied in published controlled trials for the treatment of cryptosporidiosis [92]. Like other aminoglycoside antibiotics, paromomycin hampers protein synthesis by specifically binding to the 30S ribosomal subunit and exhibits a broad spectrum of activity against bacteria and certain protozoa [119]. Animal model experiments have showed that paromomycin reduces oocyst shedding and alleviates host symptoms [18, 21, 122]. In vitro studies have further confirmed the anti-cryptosporidial properties of paromomycin [128, 185]. Prior research highlighted the effectiveness of paromomycin against cryptosporidiosis in people with AIDS [76]. While two randomized, placebo-controlled clinical trials involving individuals with HIV did not result in complete cures, they did demonstrate some degree of clinical improvement [85, 184]. In the management of pediatric patients with cryptosporidiosis and chronic diarrhea, paromomycin typically leads to improvement in certain clinical symptoms and parasitological markers, although it is often found to be less effective than nitazoxanide [92].
Other drugs
Azithromycin has demonstrated greater efficacy in the treatment of cryptosporidiosis in children compared to other medications. Studies suggest that azithromycin may exhibit therapeutic benefits in both immunocompetent and immunocompromised children, leading to rapid improvements in clinical symptoms and the clearance of parasites [12, 86, 174]. Additionally, other drugs such as mefloquine (MQ) have been studied for their potential therapeutic effects in cryptosporidiosis. In an experiment, the administration of a half-dose combination of mefloquine and NTZ resulted in significant therapeutic effects in C. parvum-infected mice. These effects included significant reductions in Cryptosporidium fecal oocyst shedding, improvements in pathological lesions in the ileum, and the normalization of levels of two cytokines, IFN-γ and IL-17 [57].
Promising Chinese herbal medicines for the future
Matrine
Matrine is a potent alkaloid extracted from Sophora flavescens [120], with a wide range of biological activities, including antibacterial, antiviral, anti-inflammatory, and immunomodulatory effects [80, 118, 204, 205]. In an experimental model of Cryptosporidium cuniculus infection, matrine has shown potential therapeutic benefits by reducing the number of oocysts shed in the host’s feces [125]. Following Cryptosporidium parvum infection of host intestinal epithelial cells, the expression of Toll-like receptors (TLRs) TLR2 and TLR4 is upregulated [192]. Matrine has been observed to inhibit the activation of these TLRs [206]. Research by Ji Rui et al. indicated that treatment with oxidized matrine led to a decrease in fecal excretion of oocysts in infected mice, significant repair of damaged intestinal villi structures, and a notable decrease in the relative expression of Toll-like receptors TLR2 and TLR4 [98]. Recent studies have shown that matrine can diminish inflammatory responses and oxidative stress by blocking the IL-6/STAT3 signaling pathway, thereby attenuating intestinal mucosal damage in rats with inflammatory bowel disease (IBD) [106]. Future investigations could explore whether matrine is able to attenuate Cryptosporidium-induced intestinal mucosal inflammation and damage through the IL-6/STAT3 signaling pathway.
Garlic
Garlic, an important species in the Allium genus, is noted for its unique medicinal properties. It contains a variety of active compounds such as phenolic compounds, saponins, and organosulfur compounds [169], known for their anti-inflammatory and antioxidant properties [55, 124]. Research has indicated that garlic holds medicinal potential in the treatment of intestinal parasitic infections [79, 102, 109]. In a study by Abouel-Nour et al., the immunological effects of Allium sativum on Cryptosporidium parvum-infected mice were investigated. The Allium sativum treatment group exhibited significantly elevated IL-5 levels and reduced IFN-γ levels, suggesting that garlic enhances the immune response and helps clear the infection [5]. Garlic is now recognized for its preventive and potentially therapeutic effects against cryptosporidiosis [54]. Gaafar demonstrated that preemptive oral administration of garlic two days before infection effectively reduced the number of Cryptosporidium oocysts in the feces and intestines of infected mice, partially normalizing intestinal structures [65]. Subsequent studies have reiterated the anti-cryptosporidial efficacy of garlic [59]. Elbahaie et al. observed the therapeutic effect of garlic by analyzing fecal samples from mice and performing examinations to count Cryptosporidium oocysts. They found that the garlic treatment group showed the highest percentage reduction in the number of oocysts, indicating the best therapeutic effect [54].
Halofuginone
Halofuginone is a synthetic derivative of febrifugine that was first isolated from Dichroa febrifuga, a traditional Chinese herb, and shows significant inhibitory activity against various protozoan parasites and cancer cells [48, 136]. It is utilized as an antiparasitic drug [207] and possesses anti-cryptosporidial properties. Research has demonstrated that, in animal models, administering moderate doses of halofuginone (100 μg/kg) from the day of infection up to 9 days post-infection as prophylaxis can reduce the severity of cryptosporidiosis, as manifested by decreased oocyst shedding, diarrhea, and mortality [150]. Additionally, the anti-cryptosporidial activity of halofuginone was verified in an in vitro assay, where the reduction in proliferation rate was observed at different time points (3, 15, 27 and 45 h post-infection). The study revealed a 68% decrease in proliferation rate at 15 hours compared to 3 h, with a more significant inhibitory effect observed at 27 and 45 h compared to 15 h [164]. In a study by Díaz et al., calf fecal samples were microscopically examined for Cryptosporidium parvum infection by antacid staining (Ziehl-Neelsen). The research revealed a significant reduction in the infection rate among halofuginone-treated calves, suggesting a preventive effect [50]. In addition, the study demonstrated that halofuginone served as an effective preventive agent against cryptosporidiosis in calves in a farm environment [47, 171, 175].
Pomegranate peel
The ripe peel of the pomegranate plant, known as pomegranate peel and belonging to the pomegranate family, is rich in antioxidant phytochemicals [11]. Pomegranate peel extract exhibits antioxidant, anti-inflammatory, antiviral, and antibacterial properties [187], along with anticoccidial activity [138]. Incorporating concentrated pomegranate extract (CPE) into the milk of newborn calves has been reported to provide resistance against Cryptosporidium parvum infection, alleviate diarrhea, and diminish intestinal symptoms associated with the infection [183]. In a study by Aboelsoued et al. [4], pomegranate peel was divided into white and red layers, both of which were treated with nanoparticles and transformed into a 3 g/mL solution of pomegranate peel in distilled water. This solution was administered to infected mice by gavage. The results indicated that both red and white pomegranate skin extracts were effective in reducing fecal oocyst excretion in infected mice, with the red skin extract showing superior efficacy. Additionally, the intestinal morphology of the mice treated with red skin extract was restored to a state resembling normal morphology. Pomegranate peel extract is rich in phenolic compounds with antioxidant properties [46]. The red skin extract, in particular, has high antioxidant activity and plays a significant role in improving biochemical markers and oxidative stress levels in Cryptosporidium parvum-infected mice, contributing to the restoration of their health status [4]. The therapeutic efficacy of pomegranate peel extract against Cryptosporidium parvum infection was further reaffirmed in a subsequent phase study [56]. To enhance the therapeutic efficacy of pomegranate peel extract, it is recommended to prioritize the red peel extract and combine it with nanoparticle technology to prepare the mixture. This formulation significantly improves absorption and utilization within the organism, thereby maximizing the therapeutic effect.
Curcumin
Curcumin, a natural polyphenolic compound extracted from turmeric root, exhibits various pharmacological effects such as antioxidant, anti-inflammatory [19, 99], and antiparasitic activities [153]. A study conducted by Sandamalie Ranasinghe et al. tested the anti-cryptosporidial properties of curcumin using human ileocecal adenocarcinoma cells (HCT-8). The results demonstrated that curcumin possessed significant anti-cryptosporidial effects with low cytotoxicity and dose-dependent efficacy [155]. Further verification of the therapeutic effects of curcumin was undertaken in a mouse model. The study revealed a significant reduction in the number of oocysts in the curcumin-treated group compared to the infected group, with a reduction rate of 74.70%. Additionally, there was an improvement in the structure of the ileocecal villi. Curcumin was also found to decrease the expression of IFN-γ and IL-18, while stimulating the antiparasitic activity of intestinal epithelial cells, thereby combating Cryptosporidium parvum infection [152]. Another independent experiment confirmed the therapeutic effects of curcumin in mice infected with Cryptosporidium parvum [19].
Conclusion
Cryptosporidiosis, a severe gastrointestinal disease caused by Cryptosporidium infection, presents a significant threat to infants, young children, and immunocompromised individuals. The absence of an effective treatment or vaccine has posed a considerable challenge to public health. Current research is progressing towards understanding molecular mechanisms, emphasizing the exploration of activated signaling pathways post-infection and their regulatory functions in host defense against the parasite. It is necessary to further develop inhibitors targeting key components of these signaling pathways to disrupt essential signaling pathways crucial for the survival of Cryptosporidium, thereby impeding its ability to infect and replicate. Additionally, ongoing investigation into host defense signaling pathways is vital to enhance host cell resistance to infection. These research directions hold great promise and offer new strategies for the treatment of Cryptosporidium infections.
Nowadays, ongoing exploration is uncovering new chemotherapeutic targets, especially Cryptosporidium-specific enzymes that play crucial roles in the parasite’s metabolic pathways and survival mechanisms. To facilitate the development of novel compounds targeting these key enzyme classes, future research should prioritize the following aspects: (1) identification of potent and low-toxicity drug compounds through high-throughput screening and structure optimization; (2) exploration of the potential for multi-target drugs and the development of combination therapies that exert multiple effective actions; and (3) investigation into the resistance mechanisms of Cryptosporidium toward existing medications and the development of new drugs capable of overcoming resistance.
The discussion also extends to Chinese herbal medicine and the ongoing research progress in clinical treatment, determining the ideal combinations and dosages, and integrating Chinese herbal medicine with conventional drugs in clinical settings. Evaluation of the combined effects in model trials may offer fresh possibilities for clinical treatment. It is believed that through continuous research efforts and advancements in technology, novel breakthroughs in the treatment of cryptosporidiosis are on the horizon.
Funding
Rui Ji was responsible for securing funding and overseeing project management. This research was supported by the Shandong Provincial Natural Science Foundation (ZR2020MH382) and the Youth Project of the Shandong Provincial Natural Science Foundation (ZR2015HQ030).
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Author contribution statement
The conception and design of this study were a collaborative effort among all authors. Yilong Lu, Xiaoning Zhang, and Zhiyu Guan jointly drafted the initial manuscript. Subsequent revisions incorporated feedback and suggestions from all contributing authors.
References
- Abaza BE, Hamza RS, Farag TI, Abdel-Hamid MA, Moustafa RA. 2016. Assessing the efficacy of Nitazoxanide in treatment of cryptosporidiosis using PCR examination. Journal of the Egyptian Society of Parasitology, 46, 683–692. [PubMed] [Google Scholar]
- Abdulla M-H, Lim K-C, Sajid M, McKerrow JH, Caffrey CR. 2007. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Medicine, 4, e14. [CrossRef] [PubMed] [Google Scholar]
- Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DHA, Newland C, Rosales-Reyes R, Kopp B, McCoy K, Montione R, Schlesinger LS, Gavrilin MA, Wewers MD, Valvano MA, Amer AO. 2011. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy, 7, 1359–1370. [CrossRef] [PubMed] [Google Scholar]
- Aboelsoued D, Abo-Aziza FAM, Mahmoud MH, Abdel Megeed KN, Abu El Ezz NMT, Abu-Salem FM. 2019. Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. Journal of Parasitic Diseases, 43, 215–228. [CrossRef] [PubMed] [Google Scholar]
- Abouel-Nour MF, EL-Shewehy DMM, Hamada SF, Morsy TA. 2015. The efficacy of three medicinal plants: garlic, ginger and mirazid and a chemical drug metronidazole against Cryptosporidium parvum. I-Immunological response. Journal of the Egyptian Society of Parasitology, 45, 559–570. [PubMed] [Google Scholar]
- Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L, Kapur V. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science, 304, 441–445. [Google Scholar]
- Abubakar I, Aliyu SH, Arumugam C, Hunter PR, Usman NK. 2007. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database of Systematic Reviews, 1, CD004932. https://doi.org/10.1002/14651858.CD004932.pub2. [Google Scholar]
- Agarwal S, Singh MK, Garg S, Chitnis CE, Singh S. 2013. Ca2+-mediated exocytosis of subtilisin-like protease 1: a key step in egress of Plasmodium falciparum merozoites. Cellular Microbiology, 15, 910–921. [CrossRef] [PubMed] [Google Scholar]
- Ahmadpour E, Safarpour H, Xiao L, Zarean M, Hatam-Nahavandi K, Barac A, Picot S, Rahimi MT, Rubino S, Mahami-Oskouei M, Spotin A, Nami S, Baghi HB. 2020. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite, 27, 27. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunology, 2, 675–680. [CrossRef] [PubMed] [Google Scholar]
- Akuru EA, Chukwuma CI, Oyeagu CE, Erukainure OL, Mashile B, Setlhodi R, Mashele SS, Makhafola TJ, Unuofin JO, Abifarin TO, Mpendulo TC. 2022. Nutritional and phytochemical profile of pomegranate (“Wonderful variety”) peel and its effects on hepatic oxidative stress and metabolic alterations. Journal of Food Biochemistry, 46, e13913. [CrossRef] [PubMed] [Google Scholar]
- Allam AF, Shehab AY. 2002. Efficacy of azithromycin, praziquantel and mirazid in treatment of cryptosporidiosis in school children. Journal of the Egyptian Society of Parasitology, 32, 969–978. [PubMed] [Google Scholar]
- Allison AC, Eugui EM. 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology, 47, 85–118. [CrossRef] [PubMed] [Google Scholar]
- Allison AC, Kowalski WJ, Muller CD, Eugui EM. 1993. Mechanisms of action of mycophenolic acid. Annals of the New York Academy of Sciences, 696, 63–87. [CrossRef] [PubMed] [Google Scholar]
- Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet, 360, 1375–1380. [CrossRef] [PubMed] [Google Scholar]
- Andersson CS, Lundgren CAK, Magnúsdóttir A, Ge C, Wieslander A, Martinez Molina D, Högbom M. 2012. The Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase: structural basis for housing lipid substrates longer than the enzyme. Structure, 20, 1062–1070. [CrossRef] [PubMed] [Google Scholar]
- Areeshi MY, Beeching NJ, Hart CA. 2007. Cryptosporidiosis in Saudi Arabia and neighboring countries. Annals of Saudi Medicine, 27, 325–332. [CrossRef] [PubMed] [Google Scholar]
- Asadpour M, Namazi F, Razavi SM, Nazifi S. 2018. Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Veterinary Parasitology, 250, 7–14. [CrossRef] [PubMed] [Google Scholar]
- Asadpour M, Namazi F, Razavi SM, Nazifi S. 2018. Curcumin: a promising treatment for Cryptosporidium parvum infection in immunosuppressed BALB/c mice. Experimental Parasitology, 195, 59–65. [CrossRef] [PubMed] [Google Scholar]
- Asma I, Sim BLH, Brent RD, Johari S, Yvonne LAL. 2015. Molecular epidemiology of Cryptosporidium in HIV/AIDS patients in Malaysia. Tropical Biomedicine, 32, 310–322. [PubMed] [Google Scholar]
- Baishanbo A, Gargala G, Duclos C, François A, Rossignol J-F, Ballet JJ, Favennec L. 2006. Efficacy of nitazoxanide and paromomycin in biliary tract cryptosporidiosis in an immunosuppressed gerbil model. Journal of Antimicrobial Chemotherapy, 57, 353–355. [CrossRef] [PubMed] [Google Scholar]
- Baragaña B, Forte B, Choi R, Nakazawa Hewitt S, Bueren-Calabuig JA, Pisco JP, Peet C, Dranow DM, Robinson DA, Jansen C, Norcross NR, Vinayak S, Anderson M, Brooks CF, Cooper CA, Damerow S, Delves M, Dowers K, Duffy J, Edwards TE, Hallyburton I, Horst BG, Hulverson MA, Ferguson L, Jiménez-Díaz MB, Jumani RS, Lorimer DD, Love MS, Maher S, Matthews H, McNamara CW, Miller P, O’Neill S, Ojo KK, Osuna-Cabello M, Pinto E, Post J, Riley J, Rottmann M, Sanz LM, Scullion P, Sharma A, Shepherd SM, Shishikura Y, Simeons FRC, Stebbins EE, Stojanovski L, Straschil U, Tamaki FK, Tamjar J, Torrie LS, Vantaux A, Witkowski B, Wittlin S, Yogavel M, Zuccotto F, Angulo-Barturen I, Sinden R, Baum J, Gamo F-J, Mäser P, Kyle DE, Winzeler EA, Myler PJ, Wyatt PG, Floyd D, Matthews D, Sharma A, Striepen B, Huston CD, Gray DW, Fairlamb AH, Pisliakov AV, Walpole C, Read KD, Van Voorhis WC, Gilbert IH. 2019. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proceedings of the National Academy of Sciences of the United States of America, 116, 7015–7020. [CrossRef] [PubMed] [Google Scholar]
- Besteiro S. 2017. Autophagy in apicomplexan parasites. Current Opinion in Microbiology, 40, 14–20. [CrossRef] [PubMed] [Google Scholar]
- Beutler B, Hoebe K, Du X, Ulevitch RJ. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. Journal of Leukocyte Biology, 74, 479–485. [CrossRef] [PubMed] [Google Scholar]
- Blackman MJ, Carruthers VB. 2013. Recent insights into apicomplexan parasite egress provide new views to a kill. Current Opinion in Microbiology, 16, 459–464. [PubMed] [Google Scholar]
- Bobak DA. 2006. Use of nitazoxanide for gastrointestinal tract infections: treatment of protozoan parasitic infection and beyond. Current Infectious Disease Reports, 8, 91–95. [CrossRef] [PubMed] [Google Scholar]
- Boehmer TK, Alden NB, Ghosh TS, Vogt RL. 2009. Cryptosporidiosis from a community swimming pool: outbreak investigation and follow-up study. Epidemiology and Infection, 137, 1651–1654. [CrossRef] [PubMed] [Google Scholar]
- Bouzid M, Kintz E, Hunter PR. 2018. Risk factors for Cryptosporidium infection in low and middle income countries: A systematic review and meta-analysis. PLoS Neglected Tropical Diseases, 12, e0006553. [CrossRef] [PubMed] [Google Scholar]
- Buckner FS, Ranade RM, Gillespie JR, Shibata S, Hulverson MA, Zhang Z, Huang W, Choi R, Verlinde CLMJ, Hol WGJ, Ochida A, Akao Y, Choy RKM, Van Voorhis WC, Arnold SLM, Jumani RS, Huston CD, Fan E. 2019. Optimization of methionyl tRNA-synthetase inhibitors for treatment of Cryptosporidium Infection. Antimicrobial Agents and Chemotherapy, 63, e02061–18. [CrossRef] [PubMed] [Google Scholar]
- Caamaño J, Hunter CA. 2002. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clinical Microbiology Reviews, 15, 414–429. [CrossRef] [PubMed] [Google Scholar]
- Caputo C, Forbes A, Frost F, Sinclair MI, Kunde TR, Hoy JF, Fairley CK. 1999. Determinants of antibodies to Cryptosporidium infection among gay and bisexual men with HIV infection. Epidemiology and Infection, 122, 291–297. [CrossRef] [PubMed] [Google Scholar]
- Castellanos-Gonzalez A, Martinez-Traverso G, Fishbeck K, Nava S, White AC. 2019. Systematic gene silencing identified Cryptosporidium nucleoside diphosphate kinase and other molecules as targets for suppression of parasite proliferation in human intestinal cells. Scientific Reports, 9, 12153. [CrossRef] [PubMed] [Google Scholar]
- Castellanos-Gonzalez A, Sadiqova A, Ortega-Mendez J, White AC. 2022. RNA-based therapy for Cryptosporidium parvum Infection: proof-of-concept studies. Infection and Immunity, 90, e0019622. [CrossRef] [PubMed] [Google Scholar]
- Castellanos-Gonzalez A, Sparks H, Nava S, Huang W, Zhang Z, Rivas K, Hulverson MA, Barrett LK, Ojo KK, Fan E, Van Voorhis WC, White AC. 2016. A novel calcium-dependent kinase inhibitor, bumped kinase inhibitor 1517, cures cryptosporidiosis in immunosuppressed mice. Journal of Infectious Diseases, 214, 1850–1855. [CrossRef] [PubMed] [Google Scholar]
- Castellanos-Gonzalez A, White AC, Ojo KK, Vidadala RSR, Zhang Z, Reid MC, Fox AMW, Keyloun KR, Rivas K, Irani A, Dann SM, Fan E, Maly DJ, Van Voorhis WC. 2013. A novel calcium-dependent protein kinase inhibitor as a lead compound for treating cryptosporidiosis. Journal of Infectious Diseases, 208, 1342–1348. [CrossRef] [PubMed] [Google Scholar]
- Centers for Disease Control, Prevention (CDC). 2006. Epidemiology of HIV/AIDS–United States, 1981–2005. MMWR. Morbidity and Mortality Weekly Report, 55, 589–592. [PubMed] [Google Scholar]
- Chadha A, Chadee K. 2021. The NF-κB pathway: Modulation by Entamoeba histolytica and other protozoan parasites. Frontiers in Cellular and Infection Microbiology, 11, 748404. [CrossRef] [PubMed] [Google Scholar]
- Chalmers RM. 2012. Waterborne outbreaks of cryptosporidiosis. Annali dell’ Istituto Superiore di Sanità, 48, 429–446. [CrossRef] [PubMed] [Google Scholar]
- Chattopadhyay S, Mahapatra RK. 2019. Identification of adaptive inhibitors of Cryptosporidium parvum fatty acyl-coenzyme A synthetase isoforms by virtual screening. Parasitology Research, 118, 3159–3171. [CrossRef] [PubMed] [Google Scholar]
- Checkley W, White AC, Jaganath D, Arrowood MJ, Chalmers RM, Chen X-M, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Priest JW, Roos DS, Striepen B, Thompson RCA, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infectious Diseases, 15, 85–94. [CrossRef] [Google Scholar]
- Chen XM, Levine SA, Splinter PL, Tietz PS, Ganong AL, Jobin C, Gores GJ, Paya CV, LaRusso NF. 2001. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology, 120, 1774–1783. [CrossRef] [PubMed] [Google Scholar]
- Chen X-M, O’Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, Limper AH, LaRusso NF. 2005. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. Journal of Immunology, 175, 7447–7456. [CrossRef] [PubMed] [Google Scholar]
- Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. 2012. Clofazimine: current status and future prospects. Journal of Antimicrobial Chemotherapy, 67, 290–298. [CrossRef] [PubMed] [Google Scholar]
- Choudhry N, Korbel DS, Edwards LA, Bajaj-Elliott M, McDonald V. 2009. Dysregulation of interferon-gamma-mediated signalling pathway in intestinal epithelial cells by Cryptosporidium parvum infection. Cellular Microbiology, 11, 1354–1364. [CrossRef] [PubMed] [Google Scholar]
- Cook WJ, Senkovich O, Hernandez A, Speed H, Chattopadhyay D. 2015. Biochemical and structural characterization of Cryptosporidium parvum lactate dehydrogenase. International Journal of Biological Macromolecules, 74, 608–619. [CrossRef] [PubMed] [Google Scholar]
- Coronado-Reyes JA, Tinoco-Salazar J, Guisa-Morales LM, Cortés-Penagos CDJ, González-Hernández JC. 2023. Obtaining polyphenolic extracts from pomegranate peel (Punica granatum) to evaluate the bactericide and antioxidant activity. Anais da Academia Brasileira de Ciencias, 95, e20200153. [CrossRef] [PubMed] [Google Scholar]
- Delafosse A, Chartier C, Dupuy MC, Dumoulin M, Pors I, Paraud C. 2015. Cryptosporidium parvum infection and associated risk factors in dairy calves in western France. Preventive Veterinary Medicine, 118, 406–412. [CrossRef] [PubMed] [Google Scholar]
- Derbyshire ER, Mazitschek R, Clardy J. 2012. Characterization of Plasmodium liver stage inhibition by halofuginone. ChemMedChem, 7, 844–849. [CrossRef] [PubMed] [Google Scholar]
- Desai AN. 2020. Cryptosporidiosis. Journal of the American Medical Association, 323, 288. [CrossRef] [PubMed] [Google Scholar]
- Díaz P, Varcasia A, Pipia AP, Tamponi C, Sanna G, Prieto A, Ruiu A, Spissu P, Díez-Baños P, Morrondo P, Scala A. 2018. Molecular characterisation and risk factor analysis of Cryptosporidium spp. in calves from Italy. Parasitology Research, 117, 3081–3090. [CrossRef] [PubMed] [Google Scholar]
- Dong S, Yang Y, Wang Y, Yang D, Yang Y, Shi Y, Li C, Li L, Chen Y, Jiang Q, Zhou Y. 2020. Prevalence of Cryptosporidium Infection in the global population: A systematic review and meta-analysis. Acta Parasitologica, 65, 882–889. [CrossRef] [PubMed] [Google Scholar]
- Doumbo O, Rossignol JF, Pichard E, Traore HA, Dembele TM, Diakite M, Traore F, Diallo DA. 1997. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. American Journal of Tropical Medicine and Hygiene, 56, 637–639. [CrossRef] [PubMed] [Google Scholar]
- El-Ashkar AM, Mahmoud S, Sabry H, Guirguis N, El Komi W, Ali E, Abu Shousha T, Abdelmksoud HF. 2022. Nitazoxanide, ivermectin, and artemether effects against cryptosporidiosis in diabetic mice: parasitological, histopathological, and chemical studies. Journal of Parasitic Diseases, 46, 1070–1079. [CrossRef] [PubMed] [Google Scholar]
- Elbahaie ES, El Gamal RL, Fathy GM, Al-Ghandour AMF, El-Akabawy N, Abd El Hameed BH, Yahia SH. 2023. The controverted therapeutic efficacy of Allium sativum and Artemisia herba-alba extracts on Cryptosporidium-infected mice. Journal of Infection in Developing Countries, 17, 732–743. [CrossRef] [PubMed] [Google Scholar]
- El-Saber Batiha G, Magdy Beshbishy A, Wasef GL, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, Taha AE, Abd-Elhakim MY, Prasad Devkota H. 2020. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients, 12, 872. [CrossRef] [PubMed] [Google Scholar]
- El-Shewehy DMM, Elshopakey GE, Ismail A, Hassan SS, Ramez AM. 2023. Therapeutic potency of ginger, garlic, and pomegranate extracts against Cryptosporidium parvum-mediated gastro-splenic damage in mice. Acta Parasitologica, 68, 32–41. [CrossRef] [PubMed] [Google Scholar]
- El-Wakil ES, Salem AE, Al-Ghandour AMF. 2021. Evaluation of possible prophylactic and therapeutic effect of mefloquine on experimental cryptosporidiosis in immunocompromised mice. Journal of Parasitic Diseases, 45, 380–393. [CrossRef] [PubMed] [Google Scholar]
- Esmat M, Abdel-Aal AA, Shalaby MA, Badawi M, Elaskary H, Yousif AB, Fahmy M-EA. 2022. Efficacy of clofazimine and nitazoxanide combination in treating intestinal cryptosporidiosis and enhancing intestinal cellular regeneration in immunocompromised mice. Food and Waterborne Parasitology, 27, e00161. [CrossRef] [PubMed] [Google Scholar]
- Farid A, Yousry M, Safwat G. 2022. Garlic (Allium sativum Linnaeus) improved inflammation and reduced cryptosporidiosis burden in immunocompromised mice. Journal of Ethnopharmacology, 292, 115174. [CrossRef] [PubMed] [Google Scholar]
- Feng X, Akiyoshi DE, Widmer G, Tzipori S. 2007. Characterization of subtilase protease in Cryptosporidium parvum and C. hominis. Journal of Parasitology, 93, 619–626. [CrossRef] [PubMed] [Google Scholar]
- Feng R, Niu Z, Zhang X, Hou W, Zhang Y, Jian F, Ning C, Zhang L, Zhang S, Wang R. 2022. Cryptosporidium parvum downregulates miR-181d in HCT-8 cells via the p50-dependent TLRs/NF-κB pathway. Veterinary Parasitology, 305, 109710. [CrossRef] [PubMed] [Google Scholar]
- Feng Y, Ryan UM, Xiao L. 2018. Genetic diversity and population structure of Cryptosporidium. Trends in Parasitology, 34, 997–1011. [CrossRef] [PubMed] [Google Scholar]
- Feng Y, Xiong Y, Qiao T, Li X, Jia L, Han Y. 2018. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Medicine, 7, 6124–6136. [CrossRef] [PubMed] [Google Scholar]
- Fournet N, Deege MP, Urbanus AT, Nichols G, Rosner BM, Chalmers RM, Gorton R, Pollock KG, van der Giessen JW, Wever PC, Dorigo-Zetsma JW, Mulder B, Mank TG, Overdevest I, Kusters JG, van Pelt WKortbeek LM. 2013. Simultaneous increase of Cryptosporidium infections in the Netherlands, the United Kingdom and Germany in late summer season, 2012. Euro Surveillance, 18, 20348. [PubMed] [Google Scholar]
- Gaafar MR. 2012. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria Journal of Medicine, 48, 59–66. [CrossRef] [Google Scholar]
- GBD 2016 Diarrhoeal Disease Collaborators. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infectious Diseases, 18, 1211–1228. [CrossRef] [Google Scholar]
- Gerace E, Lo Presti VDM, Biondo C. 2019. Cryptosporidium infection: Epidemiology, pathogenesis, and differential diagnosis. European Journal of Microbiology & Immunology, 9, 119–123. [CrossRef] [PubMed] [Google Scholar]
- Gharpure R, Perez A, Miller AD, Wikswo ME, Silver R, Hlavsa MC. 2019. Cryptosporidiosis outbreaks – United States, 2009–2017. Morbidity and Mortality Weekly Report, 68, 568–572. [CrossRef] [PubMed] [Google Scholar]
- Giridharan S, Srinivasan M. 2018. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. Journal of Inflammation Research, 11, 407–419. [CrossRef] [Google Scholar]
- Gorla SK, McNair NN, Yang G, Gao S, Hu M, Jala VR, Haribabu B, Striepen B, Cuny GD, Mead JR, Hedstrom L. 2014. Validation of IMP dehydrogenase inhibitors in a mouse model of cryptosporidiosis. Antimicrobial Agents and Chemotherapy, 58, 1603–1614. [CrossRef] [PubMed] [Google Scholar]
- Greenwood BM, Bojang K, Whitty CJM, Targett GAT. 2005. Malaria. Lancet, 365, 1487–1498. [CrossRef] [PubMed] [Google Scholar]
- Guo F, Zhang H, Fritzler JM, Rider SD, Xiang L, McNair NN, Mead JR, Zhu G. 2014. Amelioration of Cryptosporidium parvum infection in vitro and in vivo by targeting parasite fatty acyl-coenzyme A synthetases. Journal of Infectious Diseases, 209, 1279–1287. [CrossRef] [PubMed] [Google Scholar]
- Guo F, Zhang H, Payne HR, Zhu G. 2016. Differential gene expression and protein localization of Cryptosporidium parvum fatty acyl-CoA synthetase isoforms. Journal of Eukaryotic Microbiology, 63, 233–246. [CrossRef] [PubMed] [Google Scholar]
- Han D, Liu J, Chen C, Dong L, Liu Y, Chang R, Huang X, Liu Y, Wang J, Dougherty U, Bissonnette MB, Shen B, Weichselbaum RR, Xu MM, He C. 2019. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature, 566, 270–274. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Hasan MM, Stebbins EE, Choy RKM, Gillespie JR, de Hostos EL, Miller P, Mushtaq A, Ranade RM, Teixeira JE, Verlinde CLMJ, Sateriale A, Zhang Z, Osbourn DM, Griggs DW, Fan E, Buckner FS, Huston CD. 2021. Spontaneous selection of Cryptosporidium drug resistance in a calf model of infection. Antimicrobial Agents and Chemotherapy, 65, e00023–21. [CrossRef] [PubMed] [Google Scholar]
- Hashmey R, Smith NH, Cron S, Graviss EA, Chappell CL, White AC. 1997. Cryptosporidiosis in Houston, Texas. A report of 95 cases. Medicine, 76, 118–139. [CrossRef] [PubMed] [Google Scholar]
- Hawash Y, Ghonaim M, Hussein Y, Alhazmi A, Alturkistani A. 2015. Identification of Giardia lamblia and the human infectious-species of Cryptosporidium in drinking water resources in Western Saudi Arabia by nested-PCR assays. Tropical Biomedicine, 32, 216–224. [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell, 132, 344–362. [CrossRef] [PubMed] [Google Scholar]
- Hazaa IKK, Al-Taai NA, Khalil NK, Zakri AMM. 2016. Efficacy of garlic and onion oils on murin experimental Cryptosporidium parvum infection. Al-Anbar Journal of Veterinary Sciences, 9(2), 69–74. [Google Scholar]
- He X, Fang J, Huang L, Wang J, Huang X. 2015. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology, 172, 10–29. [CrossRef] [PubMed] [Google Scholar]
- Hedstrom L. 2009. IMP dehydrogenase: structure, mechanism, and inhibition. Chemical Reviews, 109, 2903–2928. [CrossRef] [PubMed] [Google Scholar]
- Hellard M, Hocking J, Willis J, Dore G, Fairley C. 2003. Risk factors leading to Cryptosporidium infection in men who have sex with men. Sexually Transmitted Infections, 79, 412–414. [CrossRef] [PubMed] [Google Scholar]
- Helmy YA, Hafez HM. 2022. Cryptosporidiosis: From prevention to treatment, a narrative review. Microorganisms, 10, 2456. [CrossRef] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. 2015. The PI3K-PKB/Akt pathway. Cold Spring Harbor Perspectives in Biology, 7, a026609. [CrossRef] [PubMed] [Google Scholar]
- Hewitt RG, Yiannoutsos CT, Higgs ES, Carey JT, Geiseler PJ, Soave R, Rosenberg R, Vazquez GJ, Wheat LJ, Fass RJ, Antoninievic Z, Walawander AL, Flanigan TP, Bender JF. 2000. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency virus infection. AIDS Clinical Trial Group. Clinical Infectious Diseases, 31, 1084–1092. [CrossRef] [PubMed] [Google Scholar]
- Hicks P, Zwiener RJ, Squires J, Savell V. 1996. Azithromycin therapy for Cryptosporidium parvum infection in four children infected with human immunodeficiency virus. Journal of Pediatrics, 129, 297–300. [CrossRef] [Google Scholar]
- Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrobial Agents and Chemotherapy, 51, 868–876. [CrossRef] [PubMed] [Google Scholar]
- Huang W, Hulverson MA, Choi R, Arnold SLM, Zhang Z, McCloskey MC, Whitman GR, Hackman RC, Rivas KL, Barrett LK, Ojo KK, Van Voorhis WC, Fan E. 2019. Development of 5-aminopyrazole-4-carboxamide-based bumped-kinase inhibitors for Cryptosporidiosis therapy. Journal of Medicinal Chemistry, 62, 3135–3146. [CrossRef] [PubMed] [Google Scholar]
- Hulverson MA, Choi R, Arnold SLM, Schaefer DA, Hemphill A, McCloskey MC, Betzer DP, Müller J, Vidadala RSR, Whitman GR, Rivas KL, Barrett LK, Hackman RC, Love MS, McNamara CW, Shaughnessy TK, Kondratiuk A, Kurnick M, Banfor PN, Lynch JJ, Freiberg GM, Kempf DJ, Maly DJ, Riggs MW, Ojo KK, Van Voorhis WC. 2017. Advances in bumped kinase inhibitors for human and animal therapy for cryptosporidiosis. International Journal for Parasitology, 47, 753–763. [CrossRef] [PubMed] [Google Scholar]
- Hulverson MA, Choi R, Vidadala RSR, Whitman GR, Vidadala VN, Ojo KK, Barrett LK, Lynch JJ, Marsh K, Kempf DJ, Maly DJ, Van Voorhis WC. 2021. Pyrrolopyrimidine bumped kinase inhibitors for the treatment of cryptosporidiosis. ACS Infectious Diseases, 7, 1200–1207. [CrossRef] [PubMed] [Google Scholar]
- Hulverson MA, Vinayak S, Choi R, Schaefer DA, Castellanos-Gonzalez A, Vidadala RSR, Brooks CF, Herbert GT, Betzer DP, Whitman GR, Sparks HN, Arnold SLM, Rivas KL, Barrett LK, White AC, Maly DJ, Riggs MW, Striepen B, Van Voorhis WC, Ojo KK. 2017. Bumped-kinase inhibitors for cryptosporidiosis therapy. Journal of Infectious Diseases, 215, 1275–1284. [CrossRef] [PubMed] [Google Scholar]
- Hussien SMM, Abdella OH, Abu-Hashim AH, Aboshiesha GA, Taha MAA, El-Shemy AS, El-Bader MM. 2013. Comparative study between the effect of nitazoxanide and paromomycine in treatment of cryptosporidiosis in hospitalized children. Journal of the Egyptian Society of Parasitology, 43, 463–470. [PubMed] [Google Scholar]
- Iroh Tam P, Arnold SLM, Barrett LK, Chen CR, Conrad TM, Douglas E, Gordon MA, Hebert D, Henrion M, Hermann D, Hollingsworth B, Houpt E, Jere KC, Lindblad R, Love MS, Makhaza L, McNamara CW, Nedi W, Nyirenda J, Operario DJ, Phulusa J, Quinnan GV, Sawyer LA, Thole H, Toto N, Winter A, Van Voorhis WC. 2021. Clofazimine for treatment of cryptosporidiosis in human immunodeficiency virus infected adults: An experimental medicine, randomized, double-blind, placebo-controlled phase 2a trial. Clinical Infectious Diseases, 73, 183–191. [CrossRef] [PubMed] [Google Scholar]
- Jafary F, Ganjalikhany MR, Moradi A, Hemati M, Jafari S. 2019. Novel peptide inhibitors for lactate dehydrogenase a (LDHA): A survey to inhibit LDHA activity via disruption of protein-protein interaction. Scientific Reports, 9, 4686. [CrossRef] [PubMed] [Google Scholar]
- Jain V, Yogavel M, Kikuchi H, Oshima Y, Hariguchi N, Matsumoto M, Goel P, Touquet B, Jumani RS, Tacchini-Cottier F, Harlos K, Huston CD, Hakimi M-A, Sharma A. 2017. Targeting Prolyl-tRNA synthetase to accelerate drug discovery against malaria, leishmaniasis, toxoplasmosis, cryptosporidiosis, and coccidiosis. Structure, 25, 1495–1505.e6. [CrossRef] [PubMed] [Google Scholar]
- Jefferies R, Yang R, Woh CK, Weldt T, Milech N, Estcourt A, Armstrong T, Hopkins R, Watt P, Reid S, Armson A, Ryan UM. 2015. Target validation of the inosine monophosphate dehydrogenase (IMPDH) gene in Cryptosporidium using Phylomer® peptides. Experimental Parasitology, 148, 40–48. [CrossRef] [PubMed] [Google Scholar]
- Ji R, Liang R, Guan Z, Li R, Fu Y, Wang H. 2018. The role of TLR4/NF-κB signaling pathway in Cryptosporidim parvum infection. Chinese Journal of Parasitology and Parasitic Diseases, 36, 361–365. [Google Scholar]
- Ji R, Liang R, Guan Z, Li R, Fu Y, Wang H. 2018. Effect of oxymatrine on Toll-like receptors on intestinal mucosa of mice infected by Cryptosporidium parvum. Chinese Journal of Parasitology and Parasitic Diseases, 36, 455–459+482. [Google Scholar]
- Jurenka JS. 2009. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative Medicine Review, 14, 141–153. [PubMed] [Google Scholar]
- Kayamba F, Faya M, Pooe OJ, Kushwaha B, Kushwaha ND, Obakachi VA, Nyamori VO, Karpoormath R. 2021. Lactate dehydrogenase and malate dehydrogenase: Potential antiparasitic targets for drug development studies. Bioorganic & Medicinal Chemistry, 50, 116458. [CrossRef] [PubMed] [Google Scholar]
- Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, Colombara DV, De Hostos EL, Engmann C, Guerrant RL, Haque R, Houpt ER, Kang G, Korpe PS, Kotloff KL, Lima AAM, Petri WA, Platts-Mills JA, Shoultz DA, Forouzanfar MH, Hay SI, Reiner RC, Mokdad AH. 2018. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Global Health, 6, e758–e768. [CrossRef] [Google Scholar]
- Khalil AM, Yasuda M, Farid AS, Desouky MI, Mohi-Eldin MM, Haridy M, Horii Y. 2015. Immunomodulatory and antiparasitic effects of garlic extract on Eimeria vermiformis-infected mice. Parasitology Research, 114, 2735–2742. [CrossRef] [PubMed] [Google Scholar]
- Khan SM, Witola WH. 2023. Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: A comprehensive review. Frontiers in Cellular and Infection Microbiology, 13, 1115522. [CrossRef] [PubMed] [Google Scholar]
- Kirubakaran S, Gorla SK, Sharling L, Zhang M, Liu X, Ray SS, Macpherson IS, Striepen B, Hedstrom L, Cuny GD. 2012. Structure-activity relationship study of selective benzimidazole-based inhibitors of Cryptosporidium parvum IMPDH. Bioorganic & Medicinal Chemistry Letters, 22, 1985–1988. [CrossRef] [PubMed] [Google Scholar]
- Klein P, Kleinová T, Volek Z, Simůnek J. 2008. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Veterinary Parasitology, 152, 53–59. [CrossRef] [PubMed] [Google Scholar]
- Kong B, Bai L, Yang L. 2024. Effect of matrine on intestinal mucosal injury in rats with inflammatory bowel disease by regulating IL-6/STAT3 signaling pathway. Journal of Guangzhou University of Traditional Chinese Medicine, 41, 1277–1284. [Google Scholar]
- Korpe PS, Gilchrist C, Burkey C, Taniuchi M, Ahmed E, Madan V, Castillo R, Ahmed S, Arju T, Alam M, Kabir M, Ahmed T, Petri WA, Haque R, Faruque ASG, Duggal P. 2019. Case-control study of Cryptosporidium transmission in Bangladeshi households. Clinical Infectious Diseases, 68, 1073–1079. [CrossRef] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet, 382, 209–222. [CrossRef] [PubMed] [Google Scholar]
- Krstin S, Sobeh M, Braun MS, Wink M. 2018. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma b. brucei and Leishmania tarentolae. Medicines, 5, 37. [CrossRef] [PubMed] [Google Scholar]
- Kumar VP, Cisneros JA, Frey KM, Castellanos-Gonzalez A, Wang Y, Gangjee A, White AC, Jorgensen WL, Anderson KS. 2014. Structural studies provide clues for analog design of specific inhibitors of Cryptosporidium hominis thymidylate synthase-dihydrofolate reductase. Bioorganic & Medicinal Chemistry Letters, 24, 4158–4161. [CrossRef] [PubMed] [Google Scholar]
- Kwakye-Nuako G, Boampong JN, Dong MK, Obiri-Yeboah D, Opoku YK, Amoako-Sakyi D, Asare KK. 2016. Modulation of cyptosporidiosis by CD4 levels in chronic diarrhoea HIV/AIDS individuals visiting Tarkwa Municipal hospital, Ghana. Asian Pacific Journal of Tropical Disease, 6, 770–775. [Google Scholar]
- Lee S, Ginese M, Beamer G, Danz HR, Girouard DJ, Chapman-Bonofiglio SP, Lee M, Hulverson MA, Choi R, Whitman GR, Ojo KK, Arnold SLM, Van Voorhis WC, Tzipori S, Therapeutic efficacy of bumped kinase inhibitor 1369 in a pig model of acute diarrhea caused by Cryptosporidium hominis, Antimicrobial Agents and Chemotherapy (2018) 62, e00147–18. [Google Scholar]
- Lendner M, Böttcher D, Delling C, Ojo KK, Van Voorhis WC, Daugschies A. 2015. A novel CDPK1 inhibitor–a potential treatment for cryptosporidiosis in calves? Parasitology Research, 114, 335–336. [CrossRef] [PubMed] [Google Scholar]
- Li M, Gong A-Y, Zhang X-T, Wang Y, Mathy NW, Martins GA, Strauss-Soukup JK, Chen X-M. 2018. Induction of a long noncoding RNA transcript, NR_045064, promotes defense gene transcription and facilitates intestinal epithelial cell responses against Cryptosporidium infection. Journal of Immunology, 201, 3630–3640. [CrossRef] [PubMed] [Google Scholar]
- Li K, Nader SM, Zhang X, Ray BC, Kim CY, Das A, Witola WH. 2019. Novel lactate dehydrogenase inhibitors with in vivo efficacy against Cryptosporidium parvum. PLoS Pathogens, 15, e1007953. [CrossRef] [PubMed] [Google Scholar]
- Li Z, Yu Q, Zhang N, Li J, Gong P, Li X, Wang X, Zhang X. 2023. Cryptosporidium parvum induces autophagy via PI3K/Akt/mTOR pathway in HCT-8 Cells. Chinese Journal of Veterinary Science, 1–7. [Google Scholar]
- Lidumniece E, Withers-Martinez C, Hackett F, Collins CR, Perrin AJ, Koussis K, Bisson C, Blackman MJ, Jirgensons A. 2021. Peptidic boronic acids are potent cell-permeable inhibitors of the malaria parasite egress serine protease SUB1. Proceedings of the National Academy of Sciences of the United States of America, 118, e2022696118. [CrossRef] [PubMed] [Google Scholar]
- Lin Y, He F, Wu L, Xu Y, Du Q. 2022. Matrine exerts pharmacological effects through multiple signaling pathways: A comprehensive review. Drug Design, Development and Therapy, 16, 533–569. [CrossRef] [Google Scholar]
- Lin J, Zhou D, Steitz TA, Polikanov YS, Gagnon MG. 2018. Ribosome-targeting antibiotics: Modes of action, mechanisms of resistance, and implications for drug design. Annual Review of Biochemistry, 87, 451–478. [CrossRef] [PubMed] [Google Scholar]
- Liu X-J, Cao M-A, Li W-H, Shen C-S, Yan S-Q, Yuan C-S. 2010. Alkaloids from Sophora flavescens Aition. Fitoterapia, 81, 524–527. [CrossRef] [PubMed] [Google Scholar]
- Liu A, Gong B, Liu X, Shen Y, Wu Y, Zhang W, Cao J. 2020. A retrospective epidemiological analysis of human Cryptosporidium infection in China during the past three decades (1987–2018). PLoS Neglected Tropical Diseases, 14, e0008146. [CrossRef] [PubMed] [Google Scholar]
- Liu M, Zhang D, Wang D, Wu X, Zhang Y, Yin J, Zhu G. 2023. Cost-effective in vivo and in vitro mouse models for evaluating anticryptosporidial drug efficacy: assessing vorinostat, docetaxel, and baicalein. Journal of Infectious Diseases, 228(10), 1430–1440. [CrossRef] [PubMed] [Google Scholar]
- Love MS, Beasley FC, Jumani RS, Wright TM, Chatterjee AK, Huston CD, Schultz PG, McNamara CW. 2017. A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis. PLoS Neglected Tropical Diseases, 11, e0005373. [CrossRef] [PubMed] [Google Scholar]
- Lu X, Li N, Qiao X, Qiu Z, Liu P. 2017. Composition analysis and antioxidant properties of black garlic extract. Journal of Food and Drug Analysis, 25:340–349. [CrossRef] [PubMed] [Google Scholar]
- Lu C, Liu X, Liu J, Tang X, Zhu G, Striepen B, Suo X. 2022. Immunocompetent rabbits infected with Cryptosporidium cuniculus as an animal model for anti-cryptosporidial drug testing. International Journal for Parasitology, 52, 205–210. [CrossRef] [PubMed] [Google Scholar]
- Madbouly N, El Amir A, Abdel Kader A, Rabee I, Farid A. 2021. The immunomodulatory activity of secnidazole-nitazoxanide in a murine cryptosporidiosis model. Journal of Medical Microbiology, 70(3), 1–12. [CrossRef] [Google Scholar]
- Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, HouptER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Global Health, 3, e564–575. [CrossRef] [Google Scholar]
- Mammeri M, Chevillot A, Thomas M, Polack B, Julien C, Marden J-P, Auclair E, Vallée I, Adjou KT. 2018. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Experimental Parasitology, 194, 1–8. [CrossRef] [PubMed] [Google Scholar]
- Manjunatha UH, Lakshminarayana SB, Jumani RS, Chao AT, Young JM, Gable JE, Knapp M, Hanna I, Galarneau J-R, Cantwell J, Kulkarni U, Turner M, Lu P, Darrell KH, Watson LC, Chan K, Patra D, Mamo M, Luu C, Cuellar C, Shaul J, Xiao L, Chen Y-B, Carney SK, Lakshman J, Osborne CS, Zambriski JA, Aziz N, Sarko C, Diagana TT. 2024. Cryptosporidium PI(4)K inhibitor EDI048 is a gut-restricted parasiticidal agent to treat paediatric enteric cryptosporidiosis. Nature Microbiology, 9, 2817–2835. [CrossRef] [PubMed] [Google Scholar]
- Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GMC, Kondreddi RR, Zou B, Gedeck P, Brooks CF, Herbert GT, Sateriale A, Tandel J, Noh S, Lakshminarayana SB, Lim SH, Goodman LB, Bodenreider C, Feng G, Zhang L, Blasco F, Wagner J, Leong FJ, Striepen B, Diagana TT. 2017. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature, 546, 376–380. [CrossRef] [PubMed] [Google Scholar]
- Marchi S, Corricelli M, Trapani E, Bravi L, Pittaro A, Delle Monache S, Ferroni L, Patergnani S, Missiroli S, Goitre L, Trabalzini L, Rimessi A, Giorgi C, Zavan B, Cassoni P, Dejana E, Retta SF, Pinton P. 2015. Defective autophagy is a key feature of cerebral cavernous malformations. EMBO Molecular Medicine, 7, 1403–1417. [CrossRef] [PubMed] [Google Scholar]
- Martucci WE, Rodriguez JM, Vargo MA, Marr M, Hamilton AD, Anderson KS. 2013. Exploring novel strategies for AIDS protozoal pathogens: α-helix mimetics targeting a key allosteric protein-protein interaction in C. hominis TS-DHFR. MedChemComm, 4. https://doi.org/10.1039/C3MD00141E [PubMed] [Google Scholar]
- Martucci WE, Udier-Blagovic M, Atreya C, Babatunde O, Vargo MA, Jorgensen WL, Anderson KS. 2009. Novel non-active site inhibitor of Cryptosporidium hominis TS-DHFR identified by a virtual screen. Bioorganic & Medicinal Chemistry Letters, 19, 418–423. [CrossRef] [PubMed] [Google Scholar]
- Masterson JE, Schwartz SD. 2014. The enzymatic reaction catalyzed by lactate dehydrogenase exhibits one dominant reaction path. Chemical Physics, 442, 132–136. [CrossRef] [Google Scholar]
- Mathy NW, Deng S, Gong A-Y, Li M, Wang Y, Burleigh O, Kochvar A, Whiteford ER, Shibata A, Chen X-M. 2022. The long non-coding RNA Nostrill regulates transcription of irf7 through interaction with nf-κb p65 to enhance intestinal epithelial defense against Cryptosporidium parvum. Frontiers in Immunology, 13, 863957. [CrossRef] [PubMed] [Google Scholar]
- McLaughlin NP, Evans P, Pines M. 2014. The chemistry and biology of febrifugine and halofuginone. Bioorganic & Medicinal Chemistry, 22, 1993–2004. [CrossRef] [PubMed] [Google Scholar]
- McNamara CW, Lee MC, Lim CS, Lim SH, Roland J, Simon O, Yeung BK, Chatterjee AK, McCormack SL, Manary MJ, Zeeman A-M, Dechering KJ, Kumar TS, Henrich PP, Gagaring K, Ibanez M, Kato N, Kuhen KL, Fischli C, Nagle A, Rottmann M, Plouffe DM, Bursulaya B, Meister S, Rameh L, Trappe J, Haasen D, Timmerman M, Sauerwein RW, Suwanarusk R, Russell B, Renia L, Nosten F, Tully DC, Kocken CH, Glynne RJ, Bodenreider C, Fidock DA, Diagana TT, Winzeler EA. 2013. Targeting Plasmodium PI(4)K to eliminate malaria. Nature, 504, 248–253. [CrossRef] [PubMed] [Google Scholar]
- Mohammed LS, Sallam EA, El Basuni SS, Eldiarby AS, Soliman MM, Aboelenin SM, Shehata SF. 2021. Ameliorative effect of neem leaf and pomegranate peel extracts in coccidial infections in New Zealand and v-line rabbits: Performance, intestinal health, oocyst shedding, carcass traits, and effect on economic measures. Animals, 11, 2441. [CrossRef] [PubMed] [Google Scholar]
- Mohebali M, Yimam Y, Woreta A. 2020. Cryptosporidium infection among people living with HIV/AIDS in Ethiopia: a systematic review and meta-analysis. Pathogens and Global Health, 114, 183–193. [CrossRef] [PubMed] [Google Scholar]
- Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BGK, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CLMJ, White AC, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Medicinal Chemistry Letters, 1, 331–335. [CrossRef] [PubMed] [Google Scholar]
- Na B-K, Kang J-M, Cheun H-I, Cho S-H, Moon S-U, Kim T-S, Sohn W-M. 2009. Cryptopain-1, a cysteine protease of Cryptosporidium parvum, does not require the pro-domain for folding. Parasitology, 136, 149–157. [CrossRef] [PubMed] [Google Scholar]
- Nava S, White AC, Castellanos-González A. 2019. Cryptosporidium parvum subtilisin-like serine protease (SUB1) is crucial for parasite egress from host cells. Infection and Immunity, 87, e00784–18. [CrossRef] [PubMed] [Google Scholar]
- Ndao M, Nath-Chowdhury M, Sajid M, Marcus V, Mashiyama ST, Sakanari J, Chow E, Mackey Z, Land KM, Jacobson MP, Kalyanaraman C, McKerrow JH, Arrowood MJ, Caffrey CR. 2013. A cysteine protease inhibitor rescues mice from a lethal Cryptosporidium parvum infection. Antimicrobial Agents and Chemotherapy, 57, 6063–6073. [CrossRef] [PubMed] [Google Scholar]
- Nguyen-Ho-Bao T, Ambe LA, Berberich M, Hermosilla C, Taubert A, Daugschies A, Kamena F. 2022. Octaarginine improves the efficacy of nitazoxanide against Cryptosporidium parvum. Pathogens, 11, 653. [CrossRef] [PubMed] [Google Scholar]
- Nime FA, Burek JD, Page DL, Holscher MA, Yardley JH. 1976. Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterology, 70, 592–598. [CrossRef] [PubMed] [Google Scholar]
- O’Hara SP, Bogert PST, Trussoni CE, Chen X, LaRusso NF. 2011. TLR4 promotes Cryptosporidium parvum clearance in a mouse model of biliary cryptosporidiosis. Journal of Parasitology, 97, 813–821. [CrossRef] [PubMed] [Google Scholar]
- Omolabi KF, Agoni C, Olotu FA, Soliman MES. 2021. Molecular basis of P131 cryptosporidial-IMPDH selectivity – structural, dynamical and mechanistic stance. Cell Biochemistry and Biophysics, 79, 11–24. [CrossRef] [PubMed] [Google Scholar]
- Palencia A, Liu R-J, Lukarska M, Gut J, Bougdour A, Touquet B, Wang E-D, Li X, Alley MRK, Freund YR, Rosenthal PJ, Hakimi M-A, Cusack S. 2016. Cryptosporidium and Toxoplasma parasites are inhibited by a benzoxaborole targeting Leucyl-tRNA synthetase. Antimicrobial Agents and Chemotherapy, 60, 5817–5827. [CrossRef] [PubMed] [Google Scholar]
- Pawlowic MC, Somepalli M, Sateriale A, Herbert GT, Gibson AR, Cuny GD, Hedstrom L, Striepen B. 2019. Genetic ablation of purine salvage in Cryptosporidium parvum reveals nucleotide uptake from the host cell. Proceedings of the National Academy of Sciences of the United States of America, 116, 21160–21165. [CrossRef] [PubMed] [Google Scholar]
- Petermann J, Paraud C, Pors I, Chartier C. 2014. Efficacy of halofuginone lactate against experimental cryptosporidiosis in goat neonates. Veterinary Parasitology, 202, 326–329. [CrossRef] [PubMed] [Google Scholar]
- Rahman SU, Gong H, Mi R, Huang Y, Han X, Chen Z. 2021. Chitosan protects immunosuppressed mice against Cryptosporidium parvum infection through TLR4/STAT1 signaling pathways and gut microbiota modulation. Frontiers in Immunology, 12, 784683. [Google Scholar]
- Rahman SU, Zhou K, Zhou S, Sun T, Mi R, Huang Y, Han X, Gong H, Chen Z. 2022. Curcumin mitigates Cryptosporidium parvum infection through modulation of gut microbiota and innate immune-related genes in immunosuppressed neonatal mice. Microbial Pathogenesis, 164, 105424. [CrossRef] [PubMed] [Google Scholar]
- Rai M, Ingle AP, Pandit R, Paralikar P, Anasane N, Santos CAD. 2020. Curcumin and curcumin-loaded nanoparticles: antipathogenic and antiparasitic activities. Expert Review of Anti-Infective Therapy, 18, 367–379. [CrossRef] [PubMed] [Google Scholar]
- Ramana CV, Gil MP, Schreiber RD, Stark GR. 2002. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends in Immunology, 23, 96–101. [CrossRef] [PubMed] [Google Scholar]
- Ranasinghe S, Zahedi A, Armson A, Lymbery AJ, Ash A. 2022. In vitro susceptibility of Cryptosporidium parvum to plant antiparasitic compounds. Pathogens, 12, 61. [CrossRef] [PubMed] [Google Scholar]
- Real E, Rodrigues L, Cabal GG, Enguita FJ, Mancio-Silva L, Mello-Vieira J, Beatty W, Vera IM, Zuzarte-Luís V, Figueira TN, Mair GR, Mota MM. 2018. Plasmodium UIS3 sequesters host LC3 to avoid elimination by autophagy in hepatocytes. Nature Microbiology, 3, 17–25. [Google Scholar]
- Rossignol J-F, Kabil SM, el-Gohary Y, Younis AM. 2006. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clinical Gastroenterology and Hepatology, 4, 320–324. [CrossRef] [PubMed] [Google Scholar]
- Rotte C, Stejskal F, Zhu G, Keithly JS, Martin W. 2001. Pyruvate: NADP+ oxidoreductase from the mitochondrion of Euglena gracilis and from the apicomplexan Cryptosporidium parvum: a biochemical relic linking pyruvate metabolism in mitochondriate and amitochondriate protists. Molecular Biology and Evolution, 18, 710–720. [CrossRef] [PubMed] [Google Scholar]
- Ruiz V, Czyzyk DJ, Valhondo M, Jorgensen WL, Anderson KS. 2019. Novel allosteric covalent inhibitors of bifunctional Cryptosporidium hominis TS-DHFR from parasitic protozoa identified by virtual screening. Bioorganic & Medicinal Chemistry Letters, 29, 1413–1418. [CrossRef] [PubMed] [Google Scholar]
- Ryan U, Hijjawi N, Xiao L. 2018. Foodborne cryptosporidiosis. International Journal for Parasitology, 48, 1–12. [CrossRef] [PubMed] [Google Scholar]
- Sarwono AEY, Mitsuhashi S, Kabir MHB, Shigetomi K, Okada T, Ohsaka F, Otsuguro S, Maenaka K, Igarashi M, Kato K, Ubukata M. 2019. Repurposing existing drugs: identification of irreversible IMPDH inhibitors by high-throughput screening. Journal of Enzyme Inhibition and Medicinal Chemistry, 34, 171–178. [CrossRef] [PubMed] [Google Scholar]
- Savioli L, Smith H, Thompson A. 2006. Giardia and Cryptosporidium join the “Neglected Diseases Initiative”. Trends in Parasitology, 22, 203–208. [CrossRef] [PubMed] [Google Scholar]
- Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SLM, Rivas KL, Vidadala RSR, Huang W, Barrett LK, Maly DJ, Fan E, Van Voorhis WC, Riggs MW. 2016. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. Journal of Infectious Diseases, 214, 1856–1864. [CrossRef] [PubMed] [Google Scholar]
- Shahiduzzaman M, Dyachenko V, Obwaller A, Unglaube S, Daugschies A. 2009. Combination of cell culture and quantitative PCR for screening of drugs against Cryptosporidium parvum. Veterinary Parasitology, 162, 271–277. [CrossRef] [PubMed] [Google Scholar]
- Shakya A, Bhat HR, Ghosh SK. 2018. Update on nitazoxanide: A multifunctional chemotherapeutic agent. Current Drug Discovery Technologies, 15, 201–213. [CrossRef] [PubMed] [Google Scholar]
- Shirley D-AT, Moonah SN, Kotloff KL. 2012. Burden of disease from cryptosporidiosis. Current Opinion in Infectious Diseases, 25, 555–563. [CrossRef] [PubMed] [Google Scholar]
- Smith RP, Chalmers RM, Mueller-Doblies D, Clifton-Hadley FA, Elwin K, Watkins J, Paiba GA, Hadfield SJ, Giles M. 2010. Investigation of farms linked to human patients with cryptosporidiosis in England and Wales. Preventive Veterinary Medicine, 94, 9–17. [CrossRef] [PubMed] [Google Scholar]
- Stark A-K, Sriskantharajah S, Hessel EM, Okkenhaug K. 2015. PI3K inhibitors in inflammation, autoimmunity and cancer. Current Opinion in Pharmacology, 23, 82–91. [CrossRef] [PubMed] [Google Scholar]
- Szychowski KA, Rybczyńska-Tkaczyk K, Gaweł-Bęben K, Świeca M, Karaś M, Jakubczyk A, Matysiak M, Binduga UE, Gmiński J. 2018. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Polish Journal of Food and Nutrition Sciences, 68, 73–81. [CrossRef] [Google Scholar]
- Toriro R, Pallett S, Woolley S, Bennett C, Hale I, Heylings J, Wilkins D, Connelly T, Muia K, Avery P, Stuart A, Morgan L, Davies M, Nevin W, Quantick O, Robinson G, Elwin K, Chalmers R, Burns D, Beeching N, Fletcher T, O’Shea M. 2024. Outbreak of diarrhea caused by a novel Cryptosporidium hominis subtype during British military training in Kenya. Open Forum Infectious Diseases, 11, ofae001. [CrossRef] [PubMed] [Google Scholar]
- Trotz-Williams LA, Jarvie BD, Peregrine AS, Duffield TF, Leslie KE. 2011. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves, Veterinary Record, 168, 509. [CrossRef] [PubMed] [Google Scholar]
- Utami WS, Murhandarwati EH, Artama WT, Kusnanto H. 2020. Cryptosporidium infection increases the risk for chronic diarrhea among people living with HIV in Southeast Asia: A systematic review and meta-analysis. Asia-Pacific Journal of Public Health, 32, 8–18. [CrossRef] [PubMed] [Google Scholar]
- Van Voorhis WC, Hulverson MA, Choi R, Huang W, Arnold SLM, Schaefer DA, Betzer DP, Vidadala RSR, Lee S, Whitman GR, Barrett LK, Maly DJ, Riggs MW, Fan E, Kennedy TJ, Tzipori S, Doggett JS, Winzer P, Anghel N, Imhof D, Müller J, Hemphill A, Ferre I, Sanchez-Sanchez R, Ortega-Mora LM, Ojo KK. 2021. One health therapeutics: Target-based drug development for cryptosporidiosis and other Apicomplexa diseases. Veterinary Parasitology, 289, 109336. [CrossRef] [PubMed] [Google Scholar]
- Vargas SL, Shenep JL, Flynn PM, Pui CH, Santana VM, Hughes WT. 1993. Azithromycin for treatment of severe Cryptosporidium diarrhea in two children with cancer. Journal of Pediatrics, 123, 154–156. [CrossRef] [Google Scholar]
- Vélez J, Lange MK, Zieger P, Yoon I, Failing K, Bauer C. 2019. Long-term use of yeast fermentation products in comparison to halofuginone for the control of cryptosporidiosis in neonatal calves. Veterinary Parasitology, 269, 57–64. [CrossRef] [PubMed] [Google Scholar]
- Viel H, Rocques H, Martin J, Chartier C. 2007. Efficacy of nitazoxanide against experimental cryptosporidiosis in goat neonates. Parasitology Research, 102, 163–166. [CrossRef] [PubMed] [Google Scholar]
- Vinayak S, Jumani RS, Miller P, Hasan MM, McLeod BI, Tandel J, Stebbins EE, Teixeira JE, Borrel J, Gonse A, Zhang M, Yu X, Wernimont A, Walpole C, Eckley S, Love MS, McNamara CW, Sharma M, Sharma A, Scherer CA, Kato N, Schreiber SL, Melillo B, Striepen v, Huston CD, Comer E. 2020. Bicyclic azetidines kill the diarrheal pathogen Cryptosporidium in mice by inhibiting parasite phenylalanyl-tRNA synthetase. Science Translational Medicine, 12, eaba8412. [CrossRef] [PubMed] [Google Scholar]
- Wang Z-D, Liu Q, Liu H-H, Li S, Zhang L, Zhao Y-K, Zhu X-Q. 2018. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasites & Vectors, 11, 28. [CrossRef] [PubMed] [Google Scholar]
- Wang C, Liu L, Zhu H, Zhang L, Wang R, Zhang Z, Huang J, Zhang S, Jian F, Ning C, Zhang L. 2019. MicroRNA expression profile of HCT-8 cells in the early phase of Cryptosporidium parvum infection. BMC Genomics, 20, 37. [CrossRef] [PubMed] [Google Scholar]
- Weber-Nordt RM, Mertelsmann R, Finke J. 1998. The JAK-STAT pathway: signal transduction involved in proliferation, differentiation and transformation. Leukemia & Lymphoma, 28, 459–467. [CrossRef] [PubMed] [Google Scholar]
- Wee P, Wang Z. 2017. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers, 9, 52. [CrossRef] [PubMed] [Google Scholar]
- Weimar JD, DiRusso CC, Delio R, Black PN. 2002. Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. Journal of Biological Chemistry, 277, 29369–29376. [CrossRef] [Google Scholar]
- Weyl-Feinstein S, Markovics A, Eitam H, Orlov A, Yishay M, Agmon R, Miron J, Izhaki I, Shabtay A. 2014. Effect of pomegranate-residue supplement on Cryptosporidium parvum oocyst shedding in neonatal calves. Journal of Dairy Science, 97, 5800–5805. [CrossRef] [PubMed] [Google Scholar]
- White AC, Chappell CL, Hayat CS, Kimball KT, Flanigan TP, Goodgame RW. 1994. Paromomycin for cryptosporidiosis in AIDS: a prospective, double-blind trial. Journal of Infectious Diseases, 170, 419–424. [CrossRef] [PubMed] [Google Scholar]
- Woolsey ID, Zeller WE, Blomstrand BM, Øines Ø, Enemark HL. 2022. Effects of selected condensed tannins on Cryptosporidium parvum growth and proliferation in HCT-8 cell cultures. Experimental Parasitology, 241, 108353. [CrossRef] [PubMed] [Google Scholar]
- Xia Z, Xu J, Lu E, He W, Deng S, Gong A-Y, Strass-Soukup J, Martins GA, Lu G, Chen X-M. 2021. m6A mRNA methylation regulates epithelial innate antimicrobial defense against cryptosporidial infection. Frontiers in Immunology, 12, 705232. [CrossRef] [PubMed] [Google Scholar]
- Xiang Q, Li M, Wen J, Ren F, Yang Z, Jiang X, Chen Y. 2022. The bioactivity and applications of pomegranate peel extract: a review. Journal of Food Biochemistry, 46, e14105. [CrossRef] [PubMed] [Google Scholar]
- Xie F, Zhang Y, Li J, Sun L, Zhang L, Qi M, Zhang S, Jian F, Li X, Li X, Ning C, Wang R. 2022. MiR-942–5p targeting the IFI27 gene regulates HCT-8 cell apoptosis via a TRAIL-dependent pathway during the early phase of Cryptosporidium parvum infection. Parasites & Vectors, 15, 291. [CrossRef] [PubMed] [Google Scholar]
- Xu QM, Fang F, Wu SH, Shi ZQ, Liu Z, Zhoa YJ, Zheng HW, Lu GX, Kong HR, Wang GJ, Ai L, Chen MX, Chen JX. 2021. Dendritic cell TLR4 induces Th1-type immune response against Cryptosporidium parvum infection. Tropical Biomedicine, 38, 172–179. [CrossRef] [PubMed] [Google Scholar]
- Yahia SH, El Gamal RL, Fathy GM, Al-Ghandour AMF, El-Akabawy N, Abdel-Hameed BH, Elbahaie ES. 2023. The potential therapeutic effect of Nigella sativa and Zingiber officinale extracts versus nitazoxanide drug against experimentally induced cryptosporidiosis in laboratory mice. Journal of Parasitic Diseases, 47, 329–339. [CrossRef] [PubMed] [Google Scholar]
- Yamanouchi K, Ishimaru T, Kakuno T, Takemoto Y, Kawatsu S, Kondo K, Maruyama M, Higaki K. 2023. Improvement and characterization of oral absorption behavior of clofazimine by SNEDDS: Quantitative evaluation of extensive lymphatic transport. European Journal of Pharmaceutics and Biopharmaceutics, 187, 141–155. [CrossRef] [PubMed] [Google Scholar]
- Yang Z, Fu Y, Gong P, Zheng J, Liu L, Yu Y, Li J, Li H, Yang J, Zhang X. 2015. Bovine TLR2 and TLR4 mediate Cryptosporidium parvum recognition in bovine intestinal epithelial cells. Microbial Pathogenesis, 85, 29–34. [CrossRef] [PubMed] [Google Scholar]
- Yang H, Zhang M, Wang X, Gong P, Zhang N, Zhang X, Li X, Li J. 2023. Cryptosporidium parvum maintains intracellular survival by activating the host cellular EGFR-PI3K/Akt signaling pathway. Molecular Immunology, 154, 69–79. [CrossRef] [PubMed] [Google Scholar]
- Yao Q, Fan Y-Y, Huang S, Hu G-R, Song J-K, Yang X, Zhao G-H. 2024. MiR-4521 affects the propagation of Cryptosporidium parvum in HCT-8 cells through targeting foxm1 by regulating cell apoptosis. Acta Tropica, 249, 107057. [CrossRef] [PubMed] [Google Scholar]
- Yin Y-L, Liu T-L, Yao Q, Wang Y-X, Wu X-M, Wang X-T, Yang X, Song J-K, Zhao G-H. 2021. Circular RNA ciRS-7 affects the propagation of Cryptosporidium parvum in HCT-8 cells by sponging miR-1270 to activate the NF-κB signaling pathway. Parasites & Vectors, 14, 238. [CrossRef] [PubMed] [Google Scholar]
- Yin J, Shen Y, Cao J. 2022. Burden of Cryptosporidium infections in the Yangtze River delta in China in the 21st century: a one health perspective. Zoonoses, 2, 993. [Google Scholar]
- Yoder JS, Beach MJ. 2010. Cryptosporidium surveillance and risk factors in the United States. Experimental Parasitology, 124, 31–39. [CrossRef] [PubMed] [Google Scholar]
- Yoon GS, Keswani RK, Sud S, Rzeczycki PM, Murashov MD, Koehn TA, Standiford TJ, Stringer KA, Rosania GR. 2016. Clofazimine biocrystal accumulation in macrophages upregulates interleukin 1 receptor antagonist production to induce a systemic anti-inflammatory state. Antimicrobial Agents and Chemotherapy, 60, 3470–3479. [CrossRef] [PubMed] [Google Scholar]
- Yuthavong Y, Kamchonwongpaisan S, Leartsakulpanich U, Chitnumsub P. 2006. Folate metabolism as a source of molecular targets for antimalarials. Future Microbiology, 1, 113–125. [CrossRef] [PubMed] [Google Scholar]
- Zahedi A, Ryan U. 2020. Cryptosporidium - An update with an emphasis on foodborne and waterborne transmission. Research in Veterinary Science, 132, 500–512. [CrossRef] [PubMed] [Google Scholar]
- Zhang H, Guo F, Zhu G. 2015. Cryptosporidium lactate dehydrogenase is associated with the parasitophorous vacuole membrane and is a potential target for developing therapeutics. PLoS Pathogens, 11, e1005250. [CrossRef] [PubMed] [Google Scholar]
- Zhang G, Huang K, Chen F, Wang J. 2009. Inhibitory effect of matrine on infection of Cryptosporidium parvum both in vivo and in vitro. Animal Husbandry & Veterinary Medicine, 41, 67–69. [Google Scholar]
- Zhang CX, Love MS, McNamara CW, Chi V, Woods AK, Joseph S, Schaefer DA, Betzer DP, Riggs MW, Iroh Tam P-Y, Van Voorhis WC, Arnold SLM. 2022. Pharmacokinetics and pharmacodynamics of clofazimine for treatment of cryptosporidiosis. Antimicrobial Agents and Chemotherapy, 66, e0156021. [CrossRef] [PubMed] [Google Scholar]
- Zhang M, Shen Y. 2018. Research progress on clinical pharmacological action of anti-hepatitis B Virus of matrine-type alkaloids. Anti-Infection Pharmacy, 15, 1–6. [Google Scholar]
- Zhang M, Shen Y. 2018. Research advances on clinical pharmacological action of anti-inflammatory agent and immunosuppressant of matrine. Anti-Infection Pharmacy, 15, 737–743. [Google Scholar]
- Zhang Y, Yan R, Hu Y. 2015. Oxymatrine inhibits lipopolysaccharide-induced inflammation by down-regulating Toll-like receptor 4/nuclear factor-kappa B in macrophages. Canadian Journal of Physiology and Pharmacology, 93, 253–260. [CrossRef] [PubMed] [Google Scholar]
- Zhang J, Yao Q, Liu Z. 2017. A novel synthesis of the efficient anti-coccidial drug halofuginone hydrobromide. Molecules, 22, 1086. [CrossRef] [PubMed] [Google Scholar]
- Zhang G, Zhang Y, Niu Z, Wang C, Xie F, Li J, Zhang S, Qi M, Jian F, Ning C, Zhang L, Wang R. 2020. Cryptosporidium parvum upregulates miR-942–5p expression in HCT-8 cells via TLR2/TLR4-NF-κB signaling. Parasites & Vectors, 13, 435. [CrossRef] [PubMed] [Google Scholar]
- Zhao N, Zhang X. 2010. Advances in the study of Nitazoxanide. Journal of Pathogen Biology, 5, 146–148+152. [Google Scholar]
- Zhou R, Gong A-Y, Chen D, Miller RE, Eischeid AN, Chen X-M. 2013. Histone deacetylases and NF-kB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. PloS One, 8, e65153. [CrossRef] [PubMed] [Google Scholar]
- Zhou R, Hu G, Liu J, Gong A-Y, Drescher KM, Chen X-M. 2009. NF-kappaB p65-dependent transactivation of miRNA genes following Cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathogens, 5, e1000681. [CrossRef] [PubMed] [Google Scholar]
- Zhou R, O’Hara SP, Chen X-M. 2011. MicroRNA regulation of innate immune responses in epithelial cells. Cellular & Molecular Immunology, 8, 371–379. [CrossRef] [PubMed] [Google Scholar]
- Zou Z, Tong F, Faergeman NJ, Børsting C, Black PN, DiRusso CC. 2003. Vectorial acylation in Saccharomyces cerevisiae. Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. Journal of Biological Chemistry, 278, 16414–16422. [CrossRef] [Google Scholar]
Cite this article as: Lu Y, Zhang X, Guan Z, Ji R, Peng F, Zhao C, Gao W & Gao F. 2025. Molecular pathogenesis of Cryptosporidium and advancements in therapeutic interventions. Parasite 32, 7. https://doi.org/10.1051/parasite/2025001.
All Tables
Anti-Cryptosporidium efficacy of different western medications in human clinical and host models.
Anti-Cryptosporidium effects of various traditional Chinese medicines in farm animals and host models.
All Figures
 |
Figure 1 Schematic diagram of the NF-κB pathway involved in the regulation of host epithelial cell RNA during Cryptosporidium infection. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


