| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 56 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/parasite/2024055 | |
| Published online | 23 September 2024 | |
Review Article
Chemotherapy for the treatment of alveolar echinococcosis: Where are we?
Chimiothérapie de l’échinococcose alvéolaire : où en sommes-nous ?
1
Université de Rennes, CHU Rennes, Inserm, EHESP, Irset (Institut de recherche en santé, environnement et travail) – UMR_S 1085, Rennes, France
2
Université de Rennes, Inserm, EHESP, Irset (Institut de recherche en santé, environnement et travail) – UMR_S 1085, Rennes, France
* Corresponding author: brice.autier@univ-rennes.fr
Received:
20
March
2024
Accepted:
23
August
2024
Alveolar echinococcosis (AE) is a severe liver disease due to infection with the Echinococcus multilocularis larval stage, called the metacestode. Management of AE is based on benzimidazole chemotherapy (albendazole or mebendazole), associated with surgery when possible. Benzimidazoles are the only compounds recommended for the treatment of AE; however, these are parasitostatic, which means that the parasite can resume growth when treatment is interrupted. Also, benzimidazoles can cause liver dysfunction which may prevent their use. Numerous drugs have been reported to have in vitro activity against E. multilocularis, but few had satisfactory in vivo activity, and none were clearly more effective than benzimidazoles. These drugs belong to various therapeutic categories including anti-infective agents (e.g. amphotericin B, mefloquine, pentamidine derivatives), anti-neoplastic compounds (e.g. imatinib, nilotinib, bortezomib), plant-extracted compounds (e.g. thymol, crocin, carvacrol) and others (e.g. metformin, verapamil, thiaclopride). These treatments are generally of limited interest due to their toxicity, their unfavorable pharmacokinetics, or the scarcity of studies involving humans. Apart from benzimidazoles, only amphotericin B, mefloquine and nitazoxanide have been reported to be used for human AE treatment, with unsatisfactory results. Few studies have aimed at developing innovative strategies for AE drug therapy, such as vectorization of drugs using nanoparticles. Altogether, this review emphasizes the urgent need for new therapeutic strategies in AE management, for which there is currently no curative chemotherapy.
Résumé
L’échinococcose alvéolaire (EA) est une maladie sévère du foie due à l’infection par la forme larvaire d’Echinococcus multilocularis, appelée métacestode. La prise en charge de l’EA repose sur la chimiothérapie par benzimidazolés (albendazole ou mébendazole), si possible associée à la chirurgie. Les benzimidazolés sont les seules molécules recommandées dans le traitement de l’EA, toutefois, ceux-ci sont parasitostatiques, ce qui signifie que le parasite peut reprendre sa croissance lors d’une interruption du traitement. Également, les benzimidazolés peuvent causer une dysfonction hépatique qui peut empêcher leur utilisation. De nombreux médicaments ont été rapportés comme ayant une activité in vitro contre E. multilocularis, mais peu d’entre eux avaient une activité in vivo satisfaisante et aucun n’était clairement plus efficace que les benzimidazolés. Ces médicaments appartiennent à diverses catégories, notamment les agents anti-infectieux (par exemple l’amphotéricine B, la méfloquine, des dérivés de la pentamidine), les composés antinéoplasiques (par exemple l’imatinib, le nilotinib, le bortézomib), les composés extraits de plantes (par exemple le thymol, la crocine, le carvacrol) et d’autres (par exemple metformine, vérapamil, thiaclopride). Ces traitements présentent généralement un intérêt limité en raison de leur toxicité, de leur pharmacocinétique défavorable ou de la rareté des études menées chez l’homme. Outre les benzimidazolés, seules l’amphotéricine B, la méfloquine et la nitazoxanide ont été utilisées dans le traitement de l’EA humaine, avec des résultats insatisfaisants. Peu d’études se sont intéressées à développer des stratégies médicamenteuses innovantes contre l’EA, comme la vectorisation de médicaments à l’aide de nanoparticules. Cette revue souligne le besoin urgent de nouvelles stratégies thérapeutiques dans la prise en charge de l’EA, pour lesquelles il n’existe pas de chimiothérapie curative.
Key words: Echinococcus multilocularis / Alveolar echinococcosis / Chemotherapy / Benzimidazole / Nanoparticles
© B. Autier et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Alveolar echinococcosis (AE) is a liver infection with the larval stage of Echinococcus multilocularis, the so-called metacestode [119]. The adult stage of this Taeniid flatworm resides in the small intestine of canids, such as foxes and dogs. This worm lays eggs, which are eliminated in the environment with the feces of the host. In its natural cycle, wild rodents, which are usual intermediate hosts, ingest eggs, which hatch in the intestine and release larvae, which than translocate across the intestinal barrier towards the liver through the portal vein, where they develop further. The larvae consist in an agglomerate of host tissues and parasitic microcysts, each one consisting in a vesicle with a liquid core, an inner layer of cells (germinative layer) and an outer acellular polysaccharidic layer (laminated layer). Humans, as aberrant intermediate hosts, become infected by ingesting eggs through contaminated water, food or hands. The vast majority of infected individuals (99%) rapidly neutralize the parasite and will not develop disease. In the remaining patients, the metacestode will indefinitely grow in the liver, leading to a cancer-like disease leading to death in the absence of treatment [31]. The infection has opportunistic behavior, as immunocompromized individuals are more at risk for developing the disease, with an accelerated course, and a higher risk of metastatic dissemination [7, 76].
The infection is diagnosed through a combination of imaging techniques [ultrasound (US), computed-tomography scanner (CT), magnetic resonance imaging (MRI)] [33], E. multilocularis-specific serology (anti-Em18 and anti-Em2+ antibodies detected by ELISA and/or western-blot as a confirmation technique) [51], and/or parasite identification (histopathology or molecular biology such as E. multilocularis-specific qPCR or DNA sequencing) [46]. Few techniques are able to assess the viability of the metacestode. Among them, anti-Em18 antibodies are of great value, as their positivity is associated with a viable parasite, but the sensitivity is imperfect as patients with small evolutive lesions can be negative for anti-Em18 [30]. Imaging techniques can be useful for the assessment of parasite viability, especially positron emission tomography (PET) imaging, which can be used to detect the metabolic activity of periparasitic immune infiltrate, which is observed for viable lesions [15]. This technique can lack sensitivity in its standard acquisition (1 h after 18 F-fluorodeoxyglucose [FDG] injection), which can be improved by delaying the acquisition to 3 h after FDG injection and/or by combining PET imaging with CT (PET/CT) or MRI (PET/MRI) [24, 32, 72, 73].
The only curative treatment of AE is surgical excision of the parasite. Imaging does not allow detection of possible small residual parasitic tissues, which can be responsible for relapse. To prevent this, surgery is associated with benzimidazole treatment, such as albendazole (10–15 mg/kg/day) or mebendazole (40–50 mg/kg/day), for at least 2 years [13]. Together with this prophylaxis, clinical and laboratory follow-up is recommended for at least 10 years, but possibly for life depending on comorbidities and the complexity of the disease [87]. If surgery is impossible, the patient will receive palliative treatment which consists of lifelong therapy with one of the benzimidazole drugs mentioned above [13]. As the development or relapse of AE is favored by immunosuppressive therapies, liver transplantation should be strictly limited to patients with severe liver dysfunction, not eligible for partial liver resection, and without metastatic lesions [13, 47]. An increasing number of AE cases are incidentally detected at an early stage of development, thanks to more available and sensitive medical imaging and US screening of at-risk populations [10, 68]. For some of them, treatment with benzimidazole only can be a therapeutic alternative, which can lead to stability or even regression of the lesion [90].
Although AE-related mortality is low in high-income countries [17], management of AE is still limited by the scarcity of therapeutic options. Also, despite the parasite being restricted to the Northern hemisphere, AE is generally considered an emerging infection, especially in Europe and North America [99]. Altogether, this emphasizes the need for a wider range of therapeutic possibilities, from repurposed drugs to innovative strategies, and the development of new compounds. Overall, it is commonly accepted that these drugs must be active on germinative cells, responsible for relapse and metastasis [75]. Given the particular nature of this slowly growing metacestode in the liver tissue, assessing drug efficacy in vitro is a difficult task, which is a hindrance to drug development. After an overview of the methods used for drug evaluation, we will review the literature about AE chemotherapy in this article.
Methods for the assessment of anti-E. multilocularis compounds
In vitro screening methods
The first in vitro evaluations of antiparasitic compounds against E. multilocularis were based on protoscoleces, obtained from fertile intermediate hosts [116], or on the in vitro culture of metacestodes, leading to the formation of parasitic vesicles [86]. Parasites were then exposed to compounds, and activity was evaluated by the occurrence of morphological alterations, the decrease in the number of vesicles or their survival, assessed by vital staining (e.g., methylene blue or eosin staining) [34, 59] or inoculation to an intermediate host [44]. With the exception of animal inoculation, these methods poorly reflect the parasitocid activity of compounds, as they do not assess their activity on germinative cells. Moreover, they rely on human eye performance, in a qualitative (morphological evaluation) or semi-quantitative (vital staining) manner, with its intrinsic subjectivity (Figure 1).
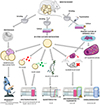 |
Figure 1 In vitro methods for the evaluation of anti-Echinococcus multilocularis compounds. In vitro methods are based on the exposure of protoscoleces, metacestodes, or cultures of germinal cells to compounds. Different techniques are used for the assessment of drug activity, including morphology and/or exclusion tests, phosphoglucoisomerase (PGI) and the Echinococcus multilocularis Alkaline Phosphatase (EmAP) assays, Em14-3-3 RT-qPCR, the Alamar Blue assay, and ATP quantification. Except for the first one, all of them rely on automated quantification of a signal. However, correlation of these signals with parasite viability remains difficult to predict. |
In order to get rid of these defects, a quantitative in vitro assay has been developed in 2010 by the team of Prof Andrew Hemphill (Vetsuiss Faculty, Bern, Switzerland): the phosphoglucoisomerase (PGI) assay. This assay relies on the indirect detection of the enzymatic activity of the EmPGI, an enzyme produced by E. multilocularis metacestode and contained in the vesicular fluid and the germinal layer [111]. This enzyme, normally restricted to the inside of the vesicle, is released in case of mechanical lysis or exposure to an active antiparasitic drug [110]. The assay consists in quantifying the EmPGI enzyme in metacestode culture supernatants after 5 to 12 days of exposure to compounds of interest. During the assay, continuous acquisition of absorbance at 340 nm by spectrophotometry allows to determine the kinetics of NADH production, which is proportional to the quantity of EmPGI. The result is expressed as % activity compared to a positive control with 100% activity (e.g. Triton X-100). This assay can be used to determine the concentration with 50% activity (EC50), and, as adaptable to 24-, 48- or 96-well plates, can be used for screening assays or structure-activity relationship assays [98]. This is currently the most frequently used in vitro assay (Figure 1). Of note, this technique does not allow the tester to evaluate the specific activity of compounds against germinative cells, as whole vesicles are used as biological material.
A similar test, based on the detection of an alkaline phosphatase released by E. multilocularis (EmAP) [112], has been used by some authors [113, 128–130]. During this assay, the metacestode culture supernatant is added to a buffer solution containing para-nitrophenylphosphate (pNPP), an alkaline phosphatase substrate. After incubation with an active compound, the metacestode releases EmAP in the supernatant, which transforms pNPP into paranitrophenol quantified by absorbance measurement at 405 nm. Drug activity is expressed as the 405 nm absorbance. While this test seems far simpler than the PGI assay, it presents the major drawback of quantifying the final product itself (instead of its kinetics of production), which is not directly related to the quantity of enzyme.
The resazurin assay (Alamar Blue assay) has also been used on metacestodes [109] or on primary cultures of germinative cells [132]. This assay is based on resazurin reduction into resofurin by mitochondria from live cells. The reaction quantification can be spectrophotometric, as both compounds have different absorption spectra, or fluorimetric, as resofurin emits at 590 nm under excitation at 530–560 nm. This technique is poorly sensitive when used on crushed metacestodes, because it does not reflect activity against germinal cells, but against all cell types of the germinative layer. On the other hand, the Alamar assay performed on primary culture of germinative cells poorly reflects the biological reality, as these cells are protected by the outer laminated layer of the metacestode [75]. As an alternative to the Alamar assay, viability of cultured germinative cells can also be assessed by quantification of ATP (CellTiter-GloTM, Promega, Madison, WI, USA).
Two studies evaluated compound activity using an RT-qPCR to quantify the expression of the Em14-3-3 gene of E. multilocularis, by comparison to the actin-coding gene as a housekeeping gene [78, 105]. As the expression of the gene reflects cellular viability, repression of Em14-3-3 expression following exposure to a compound suggests antiparasitic activity of the compound. However, this method has no longer been used for this purpose since 2008, although no clear reason has been expressed in articles from authors working on E. multilocularis [105].
In vivo methods
Activity of compounds against E. multilocularis can be evaluated in vivo, which encompasses pharmacokinetics and pharmacodynamics variables. Infection can be “primary”, by egg ingestion [114]. Infection can also be “secondary”, i.e., by inoculation of metacestodes or protoscoleces, generally in the peritoneum [114] or less frequently by subcutaneous [42] or intrahepatic inoculation [29] (Table 1). Efficiency of the treatment is usually evaluated through the weight of the lesions, sometimes through their volume or surface. As human infection is mainly hepatic, the best models are those leading to the formation of a liver metacestode, which is closer to human infection in terms of pharmacokinetics. However, these models are constraining, as they require egg isolation or surgical manipulation. Therefore, compounds are more frequently evaluated using models of secondary peritoneal AE.
Animal experimental models, advantages and drawbacks.
Active compounds
Conventional anthelminthic drugs: benzimidazoles
As previously described, management of AE relies on surgical excision of the parasite lesion, considered the only curative method, associated with pre- and post-surgery chemoprophylaxis with a benzimidazole drug, whether albendazole or mebendazole (Table 2) [13]. Activity of benzimidazoles against E. multilocularis has been widely reported, using in vitro models [40, 44, 93], in vivo models [100, 117], or in human cases [21, 127]. Benzimidazoles decreased the infectivity of in vitro exposed parasites, and stopped or slowed AE progression when given to infected mammals [54, 100, 117]. Knowledge acquired from their use in human cases also proved their efficacy by the observation of a marked increase in patient survival [21, 127]. It has been shown that patients with severe AE have a life expectancy of more than 10 years if treated, versus less than 2 years without treatment [21, 127]. However, these drugs are only parasitostatic, meaning that if the treatment is discontinued and the parasite is not surgically removed, it can resume its growth [100, 127]. Of note, some reports suggest that long-term therapy with albendazole or mebendazole (more than 2 years of treatment) can be parasitocidal, as it can result in non-viable lesions and/or the absence of recurrence after stopping therapy [4, 5, 9]. Nonetheless, stopping the therapy must involve careful lifelong follow-up to ensure that anti-Em18 antibodies and PET imaging remain negative.
Practical comparison of albendazole and mebendazole for the treatment of alveolar echinococcosis.
Benzimidazoles have a poor biodistribution profile, due to low and/or variable absorption (1–10%, increased when taken with a fatty meal) and rapid metabolization, preventing the use of most of these compounds in human medicine [21, 58, 100]. Importantly, albendazole is a pro-drug of the active albendazole sulfoxide, whereas mebendazole is active itself [22]. Among the factors that interfere with albendazole and mebendazole pharmacokinetics, enzymatic inducers (e.g., ritonavir, rifampicin, phenobarbital, carbamazepine) and inhibitors (e.g., cimetidine) are known to have an impact on their metabolization [101, 118]. These interactions are complex, as they impact the hepatic first-pass effect and metabolization of the drug and its metabolites, resulting in altered half-life and peak concentration. Lifestyle habits are also critical, especially food intake, as this impacts digestive absorption of the drug [81, 126]. Both albendazole sulfoxide and mebendazole have a high distribution volume and are mainly eliminated in the bile as further metabolites.
Benzimidazoles are inhibitors of microtubules polymerization, through binding to tubulin [57]. Their selectivity for parasitic microtubules is related to the amino acid composition of tubulin, especially the 200 residue which is a phenylalanine in susceptible helminths, instead of a tyrosine in mammals and some resistant helminths [95]. Of note, the most expressed E. multilocularis tubulin, especially by germinal metacestode cells, is Tub-2, for which the 200 residue is a tyrosine, which could explain the non-parasitocidal activity of benzimidazoles against this parasite [11, 49, 50, 103].
The activity of albendazole could be related to the host immune status. Indeed, it has been described that treatment with albendazole increases periparasite infiltration by immune cells (T CD4+ cells, B cells, plasmocytes, macrophages) and decreases the expression of FGL-2, a mediator secreted by T regulator cells (Tregs) [94]. Moreover, it has been shown that activity of albendazole was lost in athymic mice [123], suggesting that it relies at least partially on the immune system. On the contrary, albendazole treatment of infected mice reduced liver inflammation, as shown by the decrease of tissular IL-1β, IL-6, TNF-α and IFN-γ [124]. Also, in humans, immunocompromized hosts infected with E. multilocularis were reported to have a better response to albendazole treatment, with a rapid decrease of lesion size [17]. It should be kept in mind that the immune response in AE is highly complex, and repression of the immune system leads to uncontrolled parasite growth, whereas an excessive Th1/Th2 response, as observed in the late stage of the disease, is responsible for periparasitic fibrosis, limiting diffusion of drugs.
While benzimidazoles are generally well tolerated, the duration of treatment in AE patients (from 2 years to lifelong treatments) increases the risk of adverse drug reactions, such as gastrointestinal disorders, alopecia, myelosuppression (which justifies hematological monitoring), and overall, drug-induced liver injury. To prevent their occurrence and to avoid the use of suboptimal drug dosages, pharmacological monitoring of these drugs is currently recommended, but this is still too rarely available [35]. Therapeutic drug monitoring is performed by determination of plasma levels of albendazole sulfoxide (active metabolite of albendazole) or mebendazole, at peak (4 h after administration) and/or trough (before the following administration) by high-performance liquid chromatography (HPLC) [74, 136]. Liver toxicity is mainly immune-related cytolytic hepatitis; therefore, recurrence is likely in case of benzimidazole reintroduction, preventing its use [82]. Of note, cross-reactivity is not systematic, and intolerance to albendazole may be overcome by switching to mebendazole, and conversely. Also, albendazole is teratogenic if repeatedly administered during the first trimester of pregnancy [16, 25]. This can lead to complex situations of desire for pregnancy during AE treatment, as treatment discontinuation can lead to parasite growth and the hormonal status of pregnant women is suspected to favor metacestode development [6, 8].
Both albendazole and mebendazole are considered well tolerated and highly effective (i.e., at least stabilizing the disease) for the treatment of AE [87]. Albendazole use has become more prevalent over mebendazole, initially because of its lower cost and a simpler drug intake regimen (twice daily for albendazole vs three times daily for mebendazole). Consequently, most studies have focused on albendazole rather than mebendazole (in June 2024: 7,868 results when searching for “albendazole” on PubMed® vs. 2,955 results for “mebendazole”), and mebendazole has become less widely available than albendazole. Of note, the cost of both drugs increased in the last decade, but mebendazole was more affected, strengthening its place as second-line therapy [43, 60].
Salvage treatment: amphotericine B
In case of contraindications to benzimidazoles, the only available alternative is intravenous amphotericin B (AmB), either deoxycholate or liposomal, although its use is not formally recommended [13]. Its use was initially based on the observation of its in vitro parasitostatic activity, as shown by the decrease of the number of cultured vesicles. However, new vesicles are produced if the treatment is stopped [92]. In treated patients, AmB led to reduced metabolic activity at PET imaging and stabilization of lesions size [89]. The formulation seems to be important, as a colloidal dispersion of AmB was found to be inactive in a mouse model of peritoneal infection [85]. Also, an interaction was observed with albendazole, as co-exposure to both drugs led to reduced AmB activity in vitro [88]. Overall, this treatment is of limited interest in humans as contradictory outcomes have been reported, with stabilization of the disease in some patients, or disease progression in others (2/6 AE patients treated with liposomal AmB between 2008 and 2021 in a case series from the Ulm university hospital, Germany) [14, 115]. However, AmB is currently the only alternative drug therapy to benzimidazoles. Treated patients were empirically administered the lowest dosage (0.5–0.8 mg/kg of deoxycholate AmB or 3 mg/kg of liposomal AmB), with a daily dose for 10–14 days, then once to three times a week, depending on the clinical context and tolerability [14].
Other compounds
Repurposing of antiparasitic drugs used in other indications is another strategy to develop the arsenal against AE. Among the new candidate drugs for AE chemotherapy, mefloquine stands out by the number of encouraging publications [55, 56, 79, 98, 108]. This compound is already well-known for malaria prophylaxis. It has strong antiparasitic activity in vitro against E. multilocularis, with an EC50 at 30 μM [55, 108]. In animal models of AE, mefloquine was poorly effective when given orally at 25 mg/kg twice a week, but given via the intraperitoneal route or at higher doses (100 mg/kg) orally, it led to an effect similar to the current reference treatment, i.e., oral albendazole [55, 56, 98]. Treatment was not parasitocidal, as metacestodes harvested from infected mice were able to reinfect other mice after peritoneal inoculation [56]. The mechanism of action is not yet determined, but could be related to iron metabolism, energy metabolism or cellular transport [79]. To date, only two human AE cases treated with mefloquine have been reported [14, 41]. In both cases, mefloquine was combined with either albendazole or amphotericin B. One patient survived while the other died from secondary cholangitis.
In 2003, strong in vitro activity against E. multilocularis was reported for nitazoxanide [113], already used in human medicine in other indications, with an in vitro EC50 of 1.4 μM against metacestodes [54, 91, 110, 113]. However, in vivo evaluation of nitazoxanide using the mouse model of secondary peritoneal infection showed lower activity on metacestode weight compared to albendazole [114]. Its effectiveness was also evaluated in a model of primary liver infection, which showed that nitazoxanide-treated mice had significantly fewer lesions compared to the untreated group, without details concerning lesions size or weight [114]. Nitazoxanide has been used for the treatment of patients with contraindications to benzimidazole use and showed negligible activity [14, 45, 115]. Treatment with nitazoxanide led to AE stabilization in only 1/7 reported cases in the literature; the remaining 6 patients had treatment failure or discontinuation of therapy due to side effects (kidney or liver injury). This report marked the end of interest in this drug, at least in the current formulation [14, 35].
Many other compounds have been tested against E. multilocularis, with various methods, and studies have yielded variable results. They are summarized in Table 3. These drugs can be gathered into three groups: anti-infectives, anti-neoplastics, or compounds extracted from plants. Repurposing of anti-infective agents seems to be the most suitable strategy, mainly for economic reasons. Of note, antineoplastic agents are known to cause numerous side effects, as they target tumor cells with a high multiplication rate. As E. multilocularis germinal cells have a low multiplication rate, as suggested by slow disease progression, antineoplastic agents should be taken with caution, even though some of these compounds have shown in vitro activity against E. multilocularis metacestodes.
Other compounds evaluated on Echinococcus multilocularis larvae. Effective concentration at 50% (EC50) is given for in vitro tests when specified in the original publication. Otherwise, the concentration used or the minimum concentration leading to a significant effect is given. For in vivo studies, comparison with albendazole is provided, when available.
New therapeutic strategies
During the two last decades, nanoparticle-based vectorization has been recognized as a major innovation in drug administration [80]. These new formulations rely on the synthesis of 1–1,000 nm compound-containing particles, with the aim of modifying the pharmacokinetic properties. Depending on the type of nanoparticle, the drug can be linked to its surface, encapsulated into a hydrophilic or lipophilic core, or loaded into the matrix composing the nanoparticle. Three types of nanoparticles are classically described, i.e., lipid-based, polymeric, and inorganic nanoparticles [12]. Few nanoparticular therapies have been validated to date for administration to humans [83]. They are mostly used for drug vectorization (mainly antineoplastic agents); some inorganic formulations are indicated for iron supplementation, medical imaging, or photothermal therapy. Other usages are under development and evaluation, such as genetic therapy or immunotherapy [80]. Nanoparticle properties generally depend on their composition (Table 4), but many chemical methods have been developed to optimize them, for example by modifying their surface, their size, or their shape. It is also possible to address nanoparticles to targeted cells, which improves their efficacy and decreases the occurrence of side effects. With this aim, cell receptors or specific antibodies can be grafted onto the surface of nanoparticles.
Main characteristics of therapeutic nanoparticles.
Most new formulations for AE chemotherapy rely on nanoparticular vectorization of drugs [1, 23, 61, 63, 64, 96, 125]. The first report was published in 1993 and showed the efficacy of doxorubicin coupled with polyisohexylcyanoacrylate nanoparticles, in a mouse model of secondary liver infection [64]. The treatment did not modify the size of the lesions, but decreased their viability, as shown by the lower proportion of infected gerbils, Meriones unguiculatus, after inoculation of the metacestodes. In the 1990s, a study evaluated the activity of albendazole-loaded poly-D,L-lactide nanoparticles, in a mouse model of secondary liver infection [96]. The formulation was effective at low doses, but not at high doses, putatively due to toxicity on macrophages. Four studies evaluated the efficacy of liposomal albendazole, including 3 experimental studies which showed that the liposomal formulation was at least as effective as tablets of albendazole in secondary peritoneal models of infection [1, 23, 125]. The remaining study reported a series of 12 AE patients treated with liposomal albendazole in the Xinjiang district of China [61]. The authors concluded on effectiveness of the therapy in 75% of patients (9/12), and a lack of activity in 25% of patients (3/12), based on PET/CT imaging. However, no comparison to a control group treated with tablets of albendazole was carried out. Another formulation of albendazole in chitosan nanoparticles, a chitin-like polyoside, showed higher efficacy than tablets of albendazole in a mouse model [1]. Recent studies evaluated the vectorization of new drugs so-called E2-a (extracted from the Fabaceae Sophora moorcroftiana) and H1402 (carbazole aminoalcohol) using poly-lactic co-glycolic acid (PLGA) nanoparticles [62, 63]. The formulations were as effective as oral albendazole for the treatment of secondary infections (peritoneal or hepatic AE).
Other therapeutic strategies are rare and only consist of miscellaneous formulations of benzimidazoles, such as solid dispersions [28, 37, 84], and new tablet formulations containing hydroxypropyl methylcellulose [36], emulsion [104], or oily suspensions [67] for use via the oral route.
Conclusions
Benzimidazoles are the only recommended drugs for AE treatment. However, they are parasitostatic, meaning that the parasite can continue to grow in case of discontinuation. Additionally, benzimidazoles may be responsible for liver dysfunction that can prevent their use. Amphotericin B can be used as rescue treatment, but it can have severe side effects and its efficacy appears to be variable. Finally, numerous drugs have been reported to have some in vitro activity against E. multilocularis, but few had satisfactory activity in vivo, and none were clearly more effective than benzimidazoles in humans. There is an urgent need for new therapeutic strategies for the management of AE, a disease for which there is no curative chemotherapy.
Conflicts of interest
The authors have no conflicts to declare.
References
- Abulaihaiti M, Wu X-W, Qiao L, Lv H-L, Zhang H-W, Aduwayi N, Wang Y-J, Wang X-C, Peng X-Y. 2015. Efficacy of albendazole-chitosan microsphere-based treatment for alveolar echinococcosis in mice. PLoS Neglected Tropical Diseases, 9, e0003950. [CrossRef] [PubMed] [Google Scholar]
- Albani CM, Elissondo MC. 2014. Efficacy of albendazole in combination with thymol against Echinococcus multilocularis protoscoleces and metacestodes. Acta Tropica, 140, 61–67. [CrossRef] [PubMed] [Google Scholar]
- Albani CM, Pensel PE, Elissondo N, Gambino G, Elissondo MC. 2015. In vivo activity of albendazole in combination with thymol against Echinococcus multilocularis. Veterinary Parasitology, 212, 193–199. [CrossRef] [PubMed] [Google Scholar]
- Ammann RW, Fleiner-Hoffmann A, Grimm F, Eckert J. 1998. Long-term mebendazole therapy may be parasitocidal in alveolar echinococcosis. Journal of Hepatology, 29, 994–998. [CrossRef] [PubMed] [Google Scholar]
- Ammann RW, Stumpe KDM, Grimm F, Deplazes P, Huber S, Bertogg K, Fischer DR, Müllhaupt B. 2015. Outcome after discontinuing long-term benzimidazole treatment in 11 patients with non-resectable alveolar echinococcosis with negative FDG-PET/CT and Anti-EmII/3-10 serology. PLOS Neglected Tropical Diseases, 9, e0003964. [CrossRef] [PubMed] [Google Scholar]
- Antolová D, Reiterová K. 2010. Influence of Echinococcus multilocularis infection on reproduction and cellular immune response of mice. Parasite Immunology, 32, 384–387. [CrossRef] [PubMed] [Google Scholar]
- Autier B, Gottstein B, Millon L, Ramharter M, Gruener B, Bresson-Hadni S, Dion S, Robert-Gangneux F. 2023. Alveolar echinococcosis in immunocompromised hosts. Clinical Microbiology and Infection, 29, 593–599. [CrossRef] [PubMed] [Google Scholar]
- Baldolli A, Bonhomme J, Yera H, Grenouillet F, Chapon F, Barbier C, Hazera P, Verdon R. 2019. Isolated cerebral alveolar echinococcosis. Open Forum Infectious Diseases, 6, ofy349. [CrossRef] [PubMed] [Google Scholar]
- Bardonnet K, Vuitton DA, Grenouillet F, Mantion GA, Delabrousse E, Blagosklonov O, Miguet J-P, Bresson-Hadni S. 2013. 30-yr course and favorable outcome of alveolar echinococcosis despite multiple metastatic organ involvement in a non-immune suppressed patient. Annals of Clinical Microbiology and Antimicrobials, 12, 1. [CrossRef] [PubMed] [Google Scholar]
- Bartholomot G, Vuitton DA, Harraga S, Shi DZ, Giraudoux P, Barnish G, Wang YH, MacPherson CNL, Craig PS. 2002. Combined ultrasound and serologic screening for hepatic alveolar echinococcosis in central China. American Journal of Tropical Medicine and Hygiene, 66, 23–29. [CrossRef] [PubMed] [Google Scholar]
- Brehm K, Kronthaler K, Jura H, Frosch M. 2000. Cloning and characterization of β-tubulin genes from Echinococcus multilocularis. Molecular and Biochemical Parasitology, 107, 297–302. [CrossRef] [PubMed] [Google Scholar]
- Briolay T, Petithomme T, Fouet M, Nguyen-Pham N, Blanquart C, Boisgerault N. 2021. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Molecular Cancer, 20, 55. [CrossRef] [PubMed] [Google Scholar]
- Brunetti E, Kern P, Vuitton DA. 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica, 114, 1–16. [CrossRef] [PubMed] [Google Scholar]
- Burkert S, Peters L, Bloehdorn J, Grüner B. 2022. Salvage therapy for alveolar echinococcosis-a case series. Pathogens, 11, 333. [CrossRef] [PubMed] [Google Scholar]
- Caoduro C, Porot C, Vuitton DA, Bresson-Hadni S, Grenouillet F, Richou C, Boulahdour H, Blagosklonov O. 2013. The role of delayed 18F-FDG PET imaging in the follow-up of patients with alveolar echinococcosis. Journal of Nuclear Medicine, 54, 358–363. [Google Scholar]
- Carlsson G, Patring J, Ullerås E, Oskarsson A. 2011. Developmental toxicity of albendazole and its three main metabolites in zebrafish embryos. Reproductive Toxicology, 32, 129–137. [CrossRef] [Google Scholar]
- Chauchet A, Grenouillet F, Knapp J, Richou C, Delabrousse E, Dentan C, Millon L, Di Martino V, Contreras R, Deconinck E, Blagosklonov O, Vuitton DA, Bresson-Hadni S, Network FrancEchino. 2014. Increased incidence and characteristics of alveolar echinococcosis in patients with immunosuppression-associated conditions. Clinical Infectious Diseases, 59, 1095–1104. [CrossRef] [PubMed] [Google Scholar]
- Chaudhry S, Zurbriggen R, Preza M, Kämpfer T, Kaethner M, Memedovski R, Scorrano N, Hemphill A, Doggett JS, Lundström-Stadelmann B. 2022. Dual inhibition of the Echinococcus multilocularis energy metabolism. Frontiers in Veterinary Science, 9, 981664. [CrossRef] [PubMed] [Google Scholar]
- Cheng Z, Xu Z, Tian H, Liu F, Li X, Luo D, Wang Y. 2020. In vitro and in vivo efficacies of the EGFR/MEK/ERK signaling inhibitors in the treatment of alveolar echinococcosis. Antimicrobial Agents and Chemotherapy, 64, e00341-20. [CrossRef] [PubMed] [Google Scholar]
- Dang Z, Xu S, Zhang H, Gui W, Zhao Y, Duan L, Hu W. 2018. In vitro and in vivo efficacies of carbazole aminoalcohols in the treatment of alveolar echinococcosis. Acta Tropica, 185, 138–143. [CrossRef] [PubMed] [Google Scholar]
- Davis A, Pawlowski ZS, Dixon H. 1986. Multicentre clinical trials of benzimidazolecarbamates in human echinococcosis. Bulletin of the World Health Organization, 64, 383–388. [PubMed] [Google Scholar]
- Dayan AD. 2003. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Tropica, 86, 141–159. [CrossRef] [PubMed] [Google Scholar]
- Dvoroznáková E, Hrcková G, Borosková Z, Velebný S, Dubinský P. 2004. Effect of treatment with free and liposomized albendazole on selected immunological parameters and cyst growth in mice infected with Echinococcus multilocularis. Parasitology International, 53, 315–325. [CrossRef] [PubMed] [Google Scholar]
- Eberhardt N, Peters L, Kapp-Schwoerer S, Beer M, Beer AJ, Grüner B, Thaiss WM. 2022. 18F-FDG-PET/MR in alveolar echinococcosis: multiparametric imaging in a real-world setting. Pathogens, 11, 348. [CrossRef] [PubMed] [Google Scholar]
- Eckardt K, Kaltenhäuser J, Kilb C, Seiler A, Stahlmann R. 2012. Relative potency of albendazole and its sulfoxide metabolite in two in vitro tests for developmental toxicity: the rat whole embryo culture and the mouse embryonic stem cell test. Reproductive Toxicology, 34, 378–384. [CrossRef] [Google Scholar]
- Enkai S, Inaoka DK, Kouguchi H, Irie T, Yagi K, Kita K. 2020. Mitochondrial complex III in larval stage of Echinococcus multilocularis as a potential chemotherapeutic target and in vivo efficacy of atovaquone against primary hydatid cysts. Parasitology International, 75, 102004. [CrossRef] [PubMed] [Google Scholar]
- Enkai S, Kouguchi H, Inaoka DK, Irie T, Yagi K, Kita K. 2021. In vivo efficacy of combination therapy with albendazole and atovaquone against primary hydatid cysts in mice. European Journal of Clinical Microbiology & Infectious Diseases, 40, 1815–1820. [CrossRef] [PubMed] [Google Scholar]
- Fabbri J, Pensel PE, Albani CM, Lopez LM, Simonazzi A, Bermudez JM, Palma SD, Elissondo MC. 2020. Albendazole solid dispersions against alveolar echinococcosis: a pharmacotechnical strategy to improve the efficacy of the drug. Parasitology, 147, 1026–1031. [CrossRef] [PubMed] [Google Scholar]
- Gao H-J, Sun X-D, Luo Y-P, Pang H-S, Ma X-M, Zhang T, Jing T, Hu W, Shen Y-J, Cao J-P. 2021. Anti-echinococcal effect of verapamil involving the regulation of the calcium/calmodulin-dependent protein kinase II response in vitro and in a murine infection model. Parasites & Vectors, 14, 108. [CrossRef] [PubMed] [Google Scholar]
- Gottstein B, Lachenmayer A, Beldi G, Wang J, Merkle B, Vu XL, Kurath U, Müller N. 2019. Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Food and Waterborne Parasitology, 16, e00055. [CrossRef] [PubMed] [Google Scholar]
- Gottstein B, Wang J, Boubaker G, Marinova I, Spiliotis M, Müller N, Hemphill A. 2015. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis). Veterinary Parasitology, 213, 103–109. [CrossRef] [PubMed] [Google Scholar]
- Graeter T, Eberhardt N, Shi R, Schmidberger J, Beer AJ, Beer M, Henne-Bruns D, Hillenbrand A, Barth TFE, Grimm J, Kratzer W, Gruener B. 2020. Hepatic alveolar echinococcosis: correlation between computed tomography morphology and inflammatory activity in positron emission tomography. Scientific Reports, 10, 11808. [CrossRef] [PubMed] [Google Scholar]
- Graeter T, Kratzer W, Oeztuerk S, Haenle MM, Mason RA, Hillenbrand A, Kull T, Barth TF, Kern P, Gruener B. 2016. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World Journal of Gastroenterology, 22, 3621–3631. [CrossRef] [PubMed] [Google Scholar]
- Hemer S, Brehm K. 2012. In vitro efficacy of the anticancer drug imatinib on Echinococcus multilocularis larvae. International Journal of Antimicrobial Agents, 40, 458–462. [CrossRef] [PubMed] [Google Scholar]
- Hemphill A, Stadelmann B, Rufener R, Spiliotis M, Boubaker G, Müller J, Müller N, Gorgas D, Gottstein B. 2014. Treatment of echinococcosis: albendazole and mebendazole – What else? Parasite, 21, 70. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Hu C, Liu Z, Liu C, Li J, Wang Z, Xu L, Chen C, Fan H, Qian F. 2019. Enhanced oral bioavailability and anti-echinococcosis efficacy of albendazole achieved by optimizing the “spring” and “parachute”. Molecular Pharmaceutics, 16, 4978–4986. [CrossRef] [PubMed] [Google Scholar]
- Hu C, Liu Z, Liu C, Zhang Y, Fan H, Qian F. 2020. Improvement of antialveolar echinococcosis efficacy of albendazole by a novel nanocrystalline formulation with enhanced oral bioavailability. ACS Infectious Diseases, 6, 802–810. [CrossRef] [PubMed] [Google Scholar]
- Huang X, Wiehr S, Wild A-M, Voßberg P, Hoffmann W, Grüner B, Köhler C, Soboslay PT. 2018. The effects of taxanes, vorinostat and doxorubicin on growth and proliferation of Echinococcus multilocularis metacestodes assessed with magnetic resonance imaging and simultaneous positron emission tomography. Oncotarget, 9, 9073–9087. [CrossRef] [PubMed] [Google Scholar]
- Hübner C, Wiehr S, Kocherscheidt L, Wehrl H, Pichler BJ, Schmid A, Kern P, Soboslay PT. 2010. Effects of in vitro exposure of Echinococcus multilocularis metacestodes to cytostatic drugs on in vivo growth and proliferation of the parasite. Parasitology Research, 107, 459–463. [CrossRef] [PubMed] [Google Scholar]
- Ingold K, Bigler P, Thormann W, Cavaliero T, Gottstein B, Hemphill A. 1999. Efficacies of albendazole sulfoxide and albendazole sulfone against In vitro-cultivated Echinococcus multilocularis metacestodes. Antimicrobial Agents and Chemotherapy, 43, 1052–1061. [CrossRef] [PubMed] [Google Scholar]
- Jelicic K, Papic N, Viskovic K, Vince A. 2023. Case Report: Amphotericin B and mefloquine as a salvage treatment of alveolar echinococcosis. American Journal of Tropical Medicine and Hygiene, 108, 581–583. [CrossRef] [PubMed] [Google Scholar]
- Joekel DE, Lundström-Stadelmann B, Müllhaupt B, Hemphill A, Deplazes P. 2018. Evaluation of kinase-inhibitors nilotinib and everolimus against alveolar echinococcosis in vitro and in a mouse model. Experimental Parasitology, 188, 65–72. [CrossRef] [PubMed] [Google Scholar]
- Joo H, Lee J, Maskery BA, Park C, Alpern JD, Phares CR, Weinberg M, Stauffer WM. 2021. The effect of drug pricing on outpatient payments and treatment for three soil-transmitted helminth infections in the United States, 2010–2017. American Journal of Tropical Medicine and Hygiene, 104, 1851–1857. [CrossRef] [PubMed] [Google Scholar]
- Jura H, Bader A, Frosch M. 1998. In vitro activities of benzimidazoles against Echinococcus multilocularis metacestodes. Antimicrobial Agents and Chemotherapy, 42, 1052–1056. [CrossRef] [PubMed] [Google Scholar]
- Kern P, Abboud P, Kern W, Stich A, Bresson-Hadni S, Guerin B, Buttenschoen K, Gruener B, Reuter S, Hemphill A. 2008. Critical appraisal of nitazoxanide for the treatment of alveolar echinococcosis. American Journal for Tropical Medicine and Hygiene, 79, 119. [Google Scholar]
- Knapp J, Lallemand S, Monnien F, Felix S, Valmary-Degano S, Courquet S, Demonmerot F, Heyd B, Turco C, Doussot A, Bourgeois L, Bresson-Hadni S, Richou C, Millon L. 2022. Molecular diagnosis of alveolar echinococcosis in patients based on frozen and formalin-fixed paraffin-embedded tissue samples. Parasite, 29, 4. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Koch S, Bresson-Hadni S, Miguet J-P, Crumbach J-P, Gillet M, Mantion G-A, Heyd B, Vuitton D-A, Minello A, Kurtz S, Clinicians European Collaborating. 2003. Experience of liver transplantation for incurable alveolar echinococcosis: a 45-case European collaborative report. Transplantation, 75, 856–863. [CrossRef] [PubMed] [Google Scholar]
- Koike A, Becker F, Sennhenn P, Kim J, Zhang J, Hannus S, Brehm K. 2022. Targeting Echinococcus multilocularis PIM kinase for improving anti-parasitic chemotherapy. PLOS Neglected Tropical Diseases, 16, e0010483. [CrossRef] [PubMed] [Google Scholar]
- Koziol U, Brehm K. 2015. Recent advances in Echinococcus genomics and stem cell research. Veterinary Parasitology, 213, 92–102. [CrossRef] [PubMed] [Google Scholar]
- Koziol U, Rauschendorfer T, Zanon Rodríguez L, Krohne G, Brehm K. 2014. The unique stem cell system of the immortal larva of the human parasite Echinococcus multilocularis. EvoDevo, 5, 10. [CrossRef] [PubMed] [Google Scholar]
- Kronenberg PA, Deibel A, Gottstein B, Grimm F, Müllhaupt B, Meyer Zu Schwabedissen C, Aitbaev S, Omorov RA, Abdykerimov KK, Minbaeva G, Usubalieva J, Siles-Lucas M, Pepe P, Rinaldi L, Spiliotis M, Wang J, Müller N, Torgerson PR, Deplazes P. 2022. Serological assays for alveolar and cystic echinococcosis-a comparative multi-test study in Switzerland and Kyrgyzstan. Pathogens, 11, 518. [CrossRef] [PubMed] [Google Scholar]
- Küster T, Kriegel N, Boykin DW, Stephens CE, Hemphill A. 2013. In vitro and in vivo activities of dicationic diguanidino compounds against Echinococcus multilocularis metacestodes. Antimicrobial Agents and Chemotherapy, 57, 3829–3835. [CrossRef] [PubMed] [Google Scholar]
- Küster T, Lense N, Barna F, Hemphill A, Kindermann MK, Heinicke JW, Vock CA. 2012. A new promising application for highly cytotoxic metal compounds: η6-areneruthenium(II) phosphite complexes for the treatment of alveolar echinococcosis. Journal of Medicinal Chemistry, 55, 4178–4188. [CrossRef] [PubMed] [Google Scholar]
- Küster T, Stadelmann B, Aeschbacher D, Hemphill A. 2014. Activities of fenbendazole in comparison with albendazole against Echinococcus multilocularis metacestodes in vitro and in a murine infection model. International Journal of Antimicrobial Agents, 43, 335–342. [CrossRef] [PubMed] [Google Scholar]
- Küster T, Stadelmann B, Hermann C, Scholl S, Keiser J, Hemphill A. 2011. In vitro and in vivo efficacies of mefloquine-based treatment against alveolar echinococcosis. Antimicrobial Agents and Chemotherapy, 55, 713–721. [CrossRef] [PubMed] [Google Scholar]
- Küster T, Stadelmann B, Rufener R, Risch C, Müller J, Hemphill A. 2015. Oral treatments of Echinococcus multilocularis-infected mice with the antimalarial drug mefloquine that potentially interacts with parasite ferritin and cystatin. International Journal of Antimicrobial Agents, 46, 546–551. [CrossRef] [PubMed] [Google Scholar]
- Lacey E. 1990. Mode of action of benzimidazoles. Parasitology Today, 6, 112–115. [CrossRef] [Google Scholar]
- Lassègue A, Estavoyer JM, Minazzi H, Barale T, Gillet M, Vuitton D, Miguet JP. 1984. Traitement de l’échinococcose alvéolaire humaine par le flubendazole. Étude clinique, morphologique et immunologique. Gastroenterologie Clinique et Biologique, 8, 314–320. [PubMed] [Google Scholar]
- Lawton P, Walchshofer N, Sarciron ME. 2001. In vitro effects of isoprinosine and a dipeptide methyl ester on Echinococcus multilocularis protoscoleces. Journal of Helminthology, 75, 251–257. [CrossRef] [PubMed] [Google Scholar]
- Lee J, Joo H, Maskery BA, Alpern JD, Park C, Weinberg M, Stauffer WM. 2021. Increases in anti-infective drug prices, subsequent prescribing, and outpatient costs. JAMA Network Open, 4, e2113963. [CrossRef] [PubMed] [Google Scholar]
- Li H, Song T, Qin Y, Liu W, Li X, Shao Y, Wen H. 2015. Efficiency of liposomal albendazole for the treatment of the patients with complex alveolar echinococcosis: a comparative analysis of CEUS, CT, and PET/CT. Parasitology Research, 114, 4175–4180. [CrossRef] [PubMed] [Google Scholar]
- Li J, Yang Y, Han X, Li J, Tian M, Qi W, An H, Wu C, Zhang Y, Han S, Duan L, Wang W, Zhang W. 2023. Oral delivery of anti-parasitic agent-loaded PLGA nanoparticles: enhanced liver targeting and improved therapeutic effect on hepatic alveolar echinococcosis. International Journal of Nanomedicine, 18, 3069–3085. [CrossRef] [Google Scholar]
- Li Z, Zhang G, Luo Y, Gao Q, Wang J, Chen C, Xu X, Zhao Y, Li T, Ma X. 2020. In vivo effect of magnetic microspheres loaded with E2-a in the treatment of alveolar echinococcosis. Scientific Reports, 10, 12589. [CrossRef] [PubMed] [Google Scholar]
- Liance M, Nemati F, Bories C, Couvreur P. 1993. Experience with doxorubicin-bound polyisohexylcyanoacrylate nanoparticles on murine alveolar echinococcosis of the liver. International Journal for Parasitology, 23, 427–429. [CrossRef] [PubMed] [Google Scholar]
- Liu C, Fan H, Guan L, Ge R, Ma L. 2021. In vivo and in vitro efficacy of crocin against Echinococcus multilocularis. Parasites & Vectors, 14, 364. [CrossRef] [PubMed] [Google Scholar]
- Liu C, Fan H, Ma J, Ma L, Ge R-L. 2021. In vitro and in vivo efficacy of thiacloprid against Echinococcus multilocularis. Parasites & Vectors, 14, 450. [CrossRef] [PubMed] [Google Scholar]
- Liu C, Han X, Lei W, Yin J-H, Wu S-L, Zhang H-B. 2019. The efficacy of an alternative mebendazole formulation in mice infected with Echinococcus multilocularis. Acta Tropica, 196, 72–75. [CrossRef] [PubMed] [Google Scholar]
- Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H. 2014. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite, 21, 74. [PubMed] [Google Scholar]
- Liu Z, Guo X, Guo A, Zhang S, Zou Y, Wang Y, Li X, He W, Pu L, Zhang S, Zeng Q, Cai X, Wang S. 2022. HIV protease inhibitor nelfinavir is a potent drug candidate against echinococcosis by targeting Ddi1-like protein. EBioMedicine, 82, 104177. [CrossRef] [PubMed] [Google Scholar]
- Loos JA, Coccimiglio M, Nicolao MC, Rodrigues CR, Cumino AC. 2022. Metformin improves the therapeutic efficacy of low-dose albendazole against experimental alveolar echinococcosis. Parasitology, 149, 138–144. [CrossRef] [PubMed] [Google Scholar]
- Lopez LM, Pensel PE, Fabbri J, Albani CM, Elissondo N, Gambino G, Elissondo MC. 2022. The combination of carvacrol and albendazole enhanced the efficacy of monotherapy in experimental alveolar echinococcosis. Acta Tropica, 225, 106198. [CrossRef] [PubMed] [Google Scholar]
- Lötsch F, Waneck F, Auer H, Kaczirek K, Karanikas G, Ramharter M. 2017. FDG-PET/MRI in alveolar echinococcosis. International Journal of Infectious Diseases, 64, 67–68. [CrossRef] [Google Scholar]
- Lötsch F, Waneck F, Groger M, Auer H, Kaczirek K, Rausch I, Wadsak W, Hacker M, Lagler H, Ramharter M, Karanikas G. 2019. FDG-PET/MRI imaging for the management of alveolar echinococcosis: initial clinical experience at a reference centre in Austria. Tropical Medicine & International Health: TM & IH, 24, 663–670. [CrossRef] [PubMed] [Google Scholar]
- Luder PJ, Siffert B, Witassek F, Meister F, Bircher J. 1986. Treatment of hydatid disease with high oral doses of mebendazole. European Journal of Clinical Pharmacology, 31, 443–448. [CrossRef] [PubMed] [Google Scholar]
- Lundström-Stadelmann B, Rufener R, Ritler D, Zurbriggen R, Hemphill A. 2019. The importance of being parasiticidal… an update on drug development for the treatment of alveolar echinococcosis. Food and Waterborne Parasitology, 15, e00040. [CrossRef] [PubMed] [Google Scholar]
- Marquis B, Demonmerot F, Richou C, Thiéfin G, Millon L, Wallon M, Vuitton DA, Grall-Jezequel A, Grenouillet F, Epaulard O, Gervais P, Manuel O, Bresson-Hadni S, Swiss Transplant Cohort Study, FrancEchino Network. 2023. Alveolar echinococcosis in solid organ transplant recipients: a case series from two national cohorts. Parasite, 30, 9. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Mathis A, Wild P, Boettger EC, Kapel CMO, Deplazes P. 2005. Mitochondrial ribosome as the target for the macrolide antibiotic clarithromycin in the helminth Echinococcus multilocularis. Antimicrobial Agents and Chemotherapy, 49, 3251–3255. [CrossRef] [PubMed] [Google Scholar]
- Matsumoto J, Müller N, Hemphill A, Oku Y, Kamiya M, Gottstein B. 2006. 14-3-3- and II/3-10-gene expression as molecular markers to address viability and growth activity of Echinococcus multilocularis metacestodes. Parasitology, 132, 83–94. [CrossRef] [PubMed] [Google Scholar]
- Memedovski R, Preza M, Müller J, Kämpfer T, Rufener R, de Souza MVN, da Silva ET, de Andrade GF, Braga S, Uldry A-C, Buchs N, Heller M, Lundström-Stadelmann B. 2023. Investigation of the mechanism of action of mefloquine and derivatives against the parasite Echinococcus multilocularis. International Journal for Parasitology: Drugs and Drug Resistance, 21, 114–124. [CrossRef] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. 2021. Engineering precision nanoparticles for drug delivery. Nature Reviews. Drug Discovery, 20, 101–124. [CrossRef] [PubMed] [Google Scholar]
- Nagy J, Schipper HG, Koopmans RP, Butter JJ, Boxtel CJV, Kager PA. 2002. Effect of grapefruit juice or cimetidine coadministration on albendazole bioavailability. American Journal of Tropical Medicine and Hygiene, 66, 260–263. [CrossRef] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Albendazole, in LiverTox: clinical and research information on drug-induced liver injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres M del P, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin H-S. 2018. Nano based drug delivery systems: recent developments and future prospects. Journal of Nanobiotechnology, 16, 71. [CrossRef] [PubMed] [Google Scholar]
- Pensel P, Paredes A, Albani CM, Allemandi D, Sanchez Bruni S, Palma SD, Elissondo MC. 2018. Albendazole nanocrystals in experimental alveolar echinococcosis: Enhanced chemoprophylactic and clinical efficacy in infected mice. Veterinary Parasitology, 251, 78–84. [CrossRef] [PubMed] [Google Scholar]
- Porubcová J, Dvorožňáková E, Ševčíková Z. 2007. Immune response of mice to Echinococcus multilocularis infection after therapy with amphotericin B colloidal dispersion. Helminthologia, 44, 47–56. [CrossRef] [Google Scholar]
- Rausch R, Jentoft VL. 1957. Studies on the helminth fauna of Alaska. XXXI. Observations on the propagation of the larval Echinococcus multilocularis Leuckart, 1863, in vitro. Journal of Parasitology, 43, 1–8. [CrossRef] [Google Scholar]
- Reuter S, Jensen B, Buttenschoen K, Kratzer W, Kern P. 2000. Benzimidazoles in the treatment of alveolar echinococcosis: a comparative study and review of the literature. Journal of Antimicrobial Chemotherapy, 46, 451–456. [CrossRef] [Google Scholar]
- Reuter S, Beisler T, Kern P. 2010. Combined albendazole and amphotericin B against Echinococcus multilocularis in vitro. Acta Tropica, 115, 270–274. [CrossRef] [PubMed] [Google Scholar]
- Reuter S, Buck A, Grebe O, Nüssle-Kügele K, Kern P, Manfras BJ. 2003. Salvage treatment with amphotericin B in progressive human alveolar echinococcosis. Antimicrobial Agents and Chemotherapy, 47, 3586–3591. [CrossRef] [PubMed] [Google Scholar]
- Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. 2004. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology, 39, 509–517. [PubMed] [Google Scholar]
- Reuter S, Manfras B, Merkle M, Härter G, Kern P. 2006. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrobial Agents and Chemotherapy, 50, 2966–2970. [CrossRef] [PubMed] [Google Scholar]
- Reuter S, Merkle M, Brehm K, Kern P, Manfras B. 2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrobial Agents and Chemotherapy, 47, 620–625. [CrossRef] [PubMed] [Google Scholar]
- Richter D, Richter J, Grüner B, Kranz K, Franz J, Kern P. 2013. In vitro efficacy of triclabendazole and clorsulon against the larval stage of Echinococcus multilocularis. Parasitology Research, 112, 1655–1660. [CrossRef] [PubMed] [Google Scholar]
- Ricken FJ, Nell J, Grüner B, Schmidberger J, Kaltenbach T, Kratzer W, Hillenbrand A, Henne-Bruns D, Deplazes P, Moller P, Kern P, Barth TFE. 2017. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of Echinococcus multilocularis (spems) in human hepatic alveolar echinococcosis lesions. PLoS Neglected Tropical Diseases, 11, e0005636. [CrossRef] [PubMed] [Google Scholar]
- Robinson MW, McFerran N, Trudgett A, Hoey L, Fairweather I. 2004. A possible model of benzimidazole binding to beta-tubulin disclosed by invoking an inter-domain movement. Journal of Molecular Graphics & Modelling, 23, 275–284. [CrossRef] [PubMed] [Google Scholar]
- Rodrigues JM, Bories C, Emery I, Fessi H, Devissaguet JP, Liance M. 1995. Development of an injectable formulation of albendazole and in vivo evaluation of its efficacy against Echinococcus multilocularis metacestode. International Journal for Parasitology, 25, 1437–1441. [CrossRef] [PubMed] [Google Scholar]
- Rufener R, Dick L, D’Ascoli L, Ritler D, Hizem A, Wells TNC, Hemphill A, Lundström-Stadelmann B. 2018. Repurposing of an old drug: In vitro and in vivo efficacies of buparvaquone against Echinococcus multilocularis. International Journal for Parasitology: Drugs and Drug Resistance, 8, 440–450. [CrossRef] [Google Scholar]
- Rufener R, Ritler D, Zielinski J, Dick L, da Silva ET, da Silva Araujo A, Joekel DE, Czock D, Goepfert C, Moraes AM, de Souza MVN, Müller J, Mevissen M, Hemphill A, Lundström-Stadelmann B. 2018. Activity of mefloquine and mefloquine derivatives against Echinococcus multilocularis. International Journal for Parasitology. Drugs and Drug Resistance, 8, 331–340. [CrossRef] [PubMed] [Google Scholar]
- Santa MA, Umhang G, Klein C, Grant DM, Ruckstuhl KE, Musiani M, Gilleard JS, Massolo A. 2023. It’s a small world for parasites: evidence supporting the North American invasion of European Echinococcus multilocularis. Proceedings of the Royal Society B: Biological Sciences, 290, 20230128. [CrossRef] [PubMed] [Google Scholar]
- Schantz PM, Van den Bossche H, Eckert J. 1982. Chemotherapy for larval echinococcosis in animals and humans: report of a workshop. Zeitschrift für Parasitenkunde, 67, 5–26. [CrossRef] [PubMed] [Google Scholar]
- Schipper HG, Koopmans RP, Nagy J, Butter JJ, Kager PA, Van Boxtel CJ. 2000. Effect of dose increase or cimetidine co-administration on albendazole bioavailability. American Journal of Tropical Medicine and Hygiene, 63, 270–273. [CrossRef] [PubMed] [Google Scholar]
- Schubert A, Koziol U, Cailliau K, Vanderstraete M, Dissous C, Brehm K. 2014. Targeting Echinococcus multilocularis stem cells by inhibition of the Polo-like kinase EmPlk1. PLoS Neglected Tropical Diseases, 8, e2870. [CrossRef] [PubMed] [Google Scholar]
- Shi Q, Liu C, Huo L, Tao Y, Zhang H. 2022. Silencing TUBB3 expression destroys the tegument and flame cells of Echinococcus multilocularis protoscoleces. Animals, 12, 2471. [CrossRef] [PubMed] [Google Scholar]
- Shuhua X, Jiqing Y, Mingjie W, Pieying J, Fanghua G, Junjie C, Wei J, Hotez P. 2002. Augmented bioavailability and cysticidal activity of albendazole reformulated in soybean emulsion in mice infected with Echinococcus granulosus or Echinococcus multilocularis. Acta Tropica, 82, 77–84. [CrossRef] [PubMed] [Google Scholar]
- Spicher M, Naguleswaran A, Ortega-Mora LM, Müller J, Gottstein B, Hemphill A. 2008. In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes. Experimental Parasitology, 119, 475–482. [CrossRef] [PubMed] [Google Scholar]
- Spicher M, Roethlisberger C, Lany C, Stadelmann B, Keiser J, Ortega-Mora LM, Gottstein B, Hemphill A. 2008. In vitro and in vivo treatments of Echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrobial Agents and Chemotherapy, 52, 3447–3450. [CrossRef] [PubMed] [Google Scholar]
- Stadelmann B, Aeschbacher D, Huber C, Spiliotis M, Müller J, Hemphill A. 2014. Profound activity of the anti-cancer drug bortezomib against Echinococcus multilocularis metacestodes identifies the proteasome as a novel drug target for cestodes. PLoS Neglected Tropical Diseases, 8, e3352. [CrossRef] [PubMed] [Google Scholar]
- Stadelmann B, Küster T, Scholl S, Barna F, Kropf C, Keiser J, Boykin DW, Stephens CE, Hemphill A. 2011. In vitro efficacy of dicationic compounds and mefloquine enantiomers against Echinococcus multilocularis metacestodes. Antimicrobial Agents and Chemotherapy, 55, 4866–4872. [CrossRef] [PubMed] [Google Scholar]
- Stadelmann B, Rufener R, Aeschbacher D, Spiliotis M, Gottstein B, Hemphill A. 2016. Screening of the open source malaria box reveals an early lead compound for the treatment of alveolar echinococcosis. PLoS Neglected Tropical Diseases, 10, e0004535. [CrossRef] [PubMed] [Google Scholar]
- Stadelmann B, Scholl S, Müller J, Hemphill A. 2010. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. Journal of Antimicrobial Chemotherapy, 65, 512–519. [CrossRef] [PubMed] [Google Scholar]
- Stadelmann B, Spiliotis M, Müller J, Scholl S, Müller N, Gottstein B, Hemphill A. 2010. Echinococcus multilocularis phosphoglucose isomerase (EmPGI): a glycolytic enzyme involved in metacestode growth and parasite-host cell interactions. International Journal for Parasitology, 40, 1563–1574. [CrossRef] [PubMed] [Google Scholar]
- Stettler M, Siles-Lucas M, Sarciron E, Lawton P, Gottstein B, Hemphill A. 2001. Echinococcus multilocularis alkaline phosphatase as a marker for metacestode damage induced by in vitro drug treatment with albendazole sulfoxide and albendazole sulfone. Antimicrobial Agents and Chemotherapy, 45, 2256–2262. [CrossRef] [PubMed] [Google Scholar]
- Stettler M, Fink R, Walker M, Gottstein B, Geary TG, Rossignol JF, Hemphill A. 2003. In vitro parasiticidal effect of Nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrobial Agents and Chemotherapy, 47, 467–474. [CrossRef] [PubMed] [Google Scholar]
- Stettler M, Rossignol JF, Fink R, Walker M, Gottstein B, Merli M, Theurillat R, Thormann W, Dricot E, Segers R, Hemphill A. 2004. Secondary and primary murine alveolar echinococcosis: combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. International Journal for Parasitology, 34, 615–624. [PubMed] [Google Scholar]
- Tappe D, Müller A, Frosch M, Stich A. 2009. Limitations of amphotericin B and nitazoxanide in the treatment of alveolar echinococcosis. Annals of Tropical Medicine and Parasitology, 103, 177–181. [CrossRef] [PubMed] [Google Scholar]
- Taylor DH, Morris DL, Richards KS, Reffin D. 1988. Echinococcus multilocularis: in vivo results of therapy with albendazole and praziquantel. Transactions of the Royal Society of Tropical Medicine and Hygiene, 82, 611–615. [CrossRef] [PubMed] [Google Scholar]
- Vanparijs O. 1990. Chemotherapy of experimental Echinococcus multilocularis in jirds. Parasitology Research, 76, 238–240. [CrossRef] [Google Scholar]
- Venkatesan P. 1998. Albendazole. Journal of Antimicrobial Chemotherapy, 41, 145–147. [CrossRef] [Google Scholar]
- Vuitton DA, McManus DP, Rogan MT, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H, da Silva AM, Vuitton DA, McManus DP, Romig T, Rogan MR, Gottstein B, da Silva AM, Wen H, Naidich A, Tuxun T, Avcioglu A, Boufana B, Budke C, Casulli A, Güven E, Hillenbrand A, Jalousian F, Jemli MH, Knapp J, Laatamna A, Lahmar S, Naidich A, Rogan MT, Sadjjadi SM, Schmidberger J, Amri M, Bellanger A-P, Benazzouz S, Brehm K, Hillenbrand A, Jalousian F, Kachani M, Labsi M, Masala G, da Silva AM, Seyed MS, Soufli I, Touil-Boukoffa C, Wang J, Zeyhle E, Aji T, Akhan O, Bresson-Hadni S, Dziri C, Gräter T, Grüner B, Haïf A, Hillenbrand A, Koch S, Rogan MT, Tamarozzi F, Tuxun T, Giraudoux P, Torgerson P, Vizcaychipi K, Xiao N, Altintas N, Lin R, Millon L, Zhang W, Achour K, Fan H, Junghanss T, Mantion GA. 2020. International consensus on terminology to be used in the field of echinococcoses. Parasite, 27, 41. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Wang H, Zhang C-S, Fang B-B, Hou J, Li W-D, Li Z-D, Li L, Bi X-J, Li L, Abulizi A, Shao Y-M, Lin R-Y, Wen H. 2020. Dual role of hepatic macrophages in the establishment of the Echinococcus multilocularis metacestode in mice. Frontiers in Immunology, 11, 600635. [Google Scholar]
- Wang J, Goepfert C, Mueller N, Piersigilli A, Lin R, Wen H, Vuitton DA, Vuitton L, Mueller C, Gottstein B. 2018. Larval Echinococcus multilocularis infection reduces dextran sulphate sodium-induced colitis in mice by attenuating T helper type 1/type 17-mediated immune reactions. Immunology, 154, 76–88. [CrossRef] [PubMed] [Google Scholar]
- Wang J, Lin R, Zhang W, Li L, Gottstein B, Blagosklonov O, Lü G, Zhang C, Lu X, Vuitton DA, Wen H. 2014. Transcriptional profiles of cytokine/chemokine factors of immune cell-homing to the parasitic lesions: a comprehensive one-year course study in the liver of E. multilocularis-infected mice. PLoS ONE, 9, e91638. [CrossRef] [PubMed] [Google Scholar]
- Wang J, Marreros N, Rufener R, Hemphill A, Gottstein B, Lundström-Stadelmann B. 2020. Short communication: Efficacy of albendazole in Echinococcus multilocularis-infected mice depends on the functional immunity of the host. Experimental Parasitology, 219, 108013. [CrossRef] [PubMed] [Google Scholar]
- Weingartner M, Stücheli S, Jebbawi F, Gottstein B, Beldi G, Lundström-Stadelmann B, Wang J, Odermatt A. 2022. Albendazole reduces hepatic inflammation and endoplasmic reticulum-stress in a mouse model of chronic Echinococcus multilocularis infection. PLoS Neglected Tropical Diseases, 16, e0009192. [CrossRef] [PubMed] [Google Scholar]
- Wen H, New RR, Muhmut M, Wang JH, Wang YH, Zhang JH, Shao YM, Craig PS. 1996. Pharmacology and efficacy of liposome-entrapped albendazole in experimental secondary alveolar echinococcosis and effect of co-administration with cimetidine. Parasitology, 113(Pt 2), 111–121. [CrossRef] [PubMed] [Google Scholar]
- Whittaker C, Chesnais CB, Pion SDS, Kamgno J, Walker M, Basáñez M-G, Boussinesq M. 2022. Factors associated with variation in single-dose albendazole pharmacokinetics: A systematic review and modelling analysis. PLOS Neglected Tropical Diseases, 16, e0010497. [CrossRef] [PubMed] [Google Scholar]
- Wilson JF, Rausch RL, McMahon BJ, Schantz PM. 1992. Parasiticidal effect of chemotherapy in alveolar hydatid disease: review of experience with mebendazole and albendazole in Alaskan Eskimos. Clinical Infectious Diseases, 15, 234–249. [CrossRef] [PubMed] [Google Scholar]
- Xin Q, Lv W, Xu Y, Luo Y, Zhao C, Wang B, Yuan M, Li H, Song X, Jing T. 2022. 2-Deoxy-D-glucose and combined 2-Deoxy-D-glucose/albendazole exhibit therapeutic efficacy against Echinococcus granulosus protoscoleces and experimental alveolar echinococcosis. PLoS Neglected Tropical Diseases, 16, e0010618. [CrossRef] [PubMed] [Google Scholar]
- Xin Q, Yuan M, Li H, Lu J, Song X, Jing T. 2019. In vitro efficacy of ampelopsin against Echinococcus granulosus and Echinococcus multilocularis. Journal of Veterinary Medical Science, 81, 1853–1858. [CrossRef] [PubMed] [Google Scholar]
- Xin Q, Yuan M, Li H, Song X, Lu J, Jing T. 2019. In vitro and in vivo effects of 3-bromopyruvate against Echinococcus metacestodes. Veterinary Research, 50, 96. [CrossRef] [PubMed] [Google Scholar]
- Xin Q, Yuan M, Li H, Song X, Lu J, Jing T. 2020. In vitro effects of lonidamine and 6-aminonicotinamide against Echinococcus granulosus sensu stricto and Echinococcus multilocularis. Veterinary Research, 51, 29. [CrossRef] [PubMed] [Google Scholar]
- Yamashita K, Uchino J, Sato N, Furuya K, Namieno T. 1997. Establishment of a primary culture of Echinococcus multilocularis germinal cells. Journal of Gastroenterology, 32, 344–350. [CrossRef] [PubMed] [Google Scholar]
- Yang H-C, Zhang H-W, Yang J, Liu S-W, Zhang S-J. 2022. Autocrine osteopontin is involved in maintaining the growth and metastasis of Echinococcus multilocularis. Acta Tropica, 228, 106328. [CrossRef] [PubMed] [Google Scholar]
- Yarahmadov T, Wang J, Sanchez-Taltavull D, Rojas CAA, Brodie T, Büchi I, Keogh A, Gottstein B, Stroka D, Beldi G. 2022. Primary infection by E. multilocularis induces distinct patterns of cross talk between hepatic natural killer T cells and regulatory T cells in mice. Infection and Immunity, 90, e0017422. [CrossRef] [PubMed] [Google Scholar]
- Yuan M, Luo Y, Xin Q, Gao H, Zhang G, Jing T. 2016. Efficacy of osthole for Echinococcus granulosus in vitro and Echinococcus multilocularis in vivo. Veterinary Parasitology, 226, 38–43. [CrossRef] [PubMed] [Google Scholar]
- Zeugin T, Zysset T, Cotting J. 1990. Therapeutic monitoring of albendazole: a high-performance liquid chromatography method for determination of its active metabolite albendazole sulfoxide. Therapeutic Drug Monitoring, 12, 187. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Autier B, Robert-Gangneux F & Dion S. 2024. Chemotherapy for the treatment of alveolar echinococcosis: Where are we? Parasite 31, 56.
All Tables
Practical comparison of albendazole and mebendazole for the treatment of alveolar echinococcosis.
Other compounds evaluated on Echinococcus multilocularis larvae. Effective concentration at 50% (EC50) is given for in vitro tests when specified in the original publication. Otherwise, the concentration used or the minimum concentration leading to a significant effect is given. For in vivo studies, comparison with albendazole is provided, when available.
All Figures
 |
Figure 1 In vitro methods for the evaluation of anti-Echinococcus multilocularis compounds. In vitro methods are based on the exposure of protoscoleces, metacestodes, or cultures of germinal cells to compounds. Different techniques are used for the assessment of drug activity, including morphology and/or exclusion tests, phosphoglucoisomerase (PGI) and the Echinococcus multilocularis Alkaline Phosphatase (EmAP) assays, Em14-3-3 RT-qPCR, the Alamar Blue assay, and ATP quantification. Except for the first one, all of them rely on automated quantification of a signal. However, correlation of these signals with parasite viability remains difficult to predict. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


