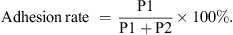| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 18 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/parasite/2024014 | |
| Published online | 26 March 2024 | |
Research Article
Unraveling the pathogenic potential of the Pentatrichomonas hominis PHGD strain: impact on IPEC-J2 cell growth, adhesion, and gene expression
Découvrir le potentiel pathogène de la souche PHGD de Pentatrichomonas hominis : impact sur la croissance, l'adhésion et l'expression des gènes des cellules IPEC-J2
1
Key Laboratory of Livestock Disease Prevention of Guangdong Province, Key Laboratory of Avian Influenza and Other Major Poultry Diseases Prevention and Control, Ministry of Agriculture and Rural Affairs, Institute of Animal Health, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, China
2
Wen’s Group Academy, Wen’s Foodstuffs Group Co., Ltd., Xinxing, Guangdong 527400, China
3
Guangdong Jingjie Inspection and Testing Co., Ltd., Xinxing, Guangdong 527400, China
* Corresponding author: smf7810@126.com; lsq6969@163.com
Received:
6
November
2023
Accepted:
27
February
2024
Pentatrichomonas hominis, a flagellated parasitic protozoan, predominantly infects the mammalian digestive tract, often causing symptoms such as abdominal pain and diarrhea. However, studies investigating its pathogenicity are limited, and the mechanisms underlying P. hominis-induced diarrhea remain unclear. Establishing an in vitro cell model for P. hominis infection is imperative. This study investigated the interaction between P. hominis and IPEC-J2 cells and its impact on parasite growth, adhesion, morphology, and cell viability. Co-cultivation of P. hominis with IPEC-J2 cells resulted in exponential growth of the parasite, with peak densities reaching approximately 4.8 × 105 cells/mL and 1.2 × 106 cells/mL at 48 h for initial inoculation concentrations of 104 cells/mL and 105 cells/mL, respectively. The adhesion rate of P. hominis to IPEC-J2 cells reached a maximum of 93.82% and 86.57% at 24 h for initial inoculation concentrations of 104 cells/mL and 105 cells/mL, respectively. Morphological changes in IPEC-J2 cells co-cultivated with P. hominis were observed, manifesting as elongated and irregular shapes. The viability of IPEC-J2 cells exhibited a decreasing trend with increasing P. hominis concentration and co-cultivation time. Additionally, the mRNA expression levels of IL-6, IL-8, and TNF-α were upregulated, whereas those of CAT and CuZn-SOD were downregulated. These findings provide quantitative evidence that P. hominis can promote its growth by adhering to IPEC-J2 cells, inducing morphological changes, reducing cell viability, and triggering inflammatory responses. Further in vivo studies are warranted to confirm these results and enhance our understanding of P. hominis infection.
Résumé
Pentatrichomonas hominis, un protozoaire parasite flagellé, infecte principalement le tube digestif des mammifères, provoquant souvent des symptômes tels que des douleurs abdominales et de la diarrhée. Cependant, les études portant sur sa pathogénicité sont limitées et les mécanismes sous-jacents à la diarrhée induite par P. hominis restent flous. L’établissement d’un modèle cellulaire in vitro de l’infection à P. hominis est impératif. Cette étude a examiné l’interaction entre P. hominis et les cellules IPEC-J2 et son impact sur la croissance du parasite, l’adhésion, la morphologie et la viabilité cellulaire. La co-culture de P. hominis avec des cellules IPEC-J2 a entraîné une croissance exponentielle du parasite, avec des densités maximales atteignant environ 4,8 × 105 cellules/mL et 1,2 × 106 cellules/mL à 48 h pour des concentrations d’inoculation initiales de 104 cellules/mL et 105 cellules/mL, respectivement. Le taux d’adhésion de P. hominis aux cellules IPEC-J2 a atteint un maximum de 93,82 % et 86,57 % après 24 h pour des concentrations d’inoculation initiales de 104 cellules/mL et 105 cellules/mL, respectivement. Des changements morphologiques dans les cellules IPEC-J2 co-cultivées avec P. hominis ont été observés, se manifestant par des formes allongées et irrégulières. La viabilité des cellules IPEC-J2 a montré une tendance à la baisse avec l’augmentation de la concentration de P. hominis et de la durée de co-culture. De plus, les niveaux d’expression d’ARNm d’IL-6, d’IL-8 et de TNF-α étaient régulés positivement, tandis que ceux de CAT et de CuZn-SOD étaient régulés négativement. Ces résultats fournissent des preuves quantitatives que P. hominis peut favoriser sa croissance en adhérant aux cellules IPEC-J2, en induisant des changements morphologiques, en réduisant la viabilité cellulaire et en déclenchant des réponses inflammatoires. D’autres études in vivo sont nécessaires pour confirmer ces résultats et améliorer notre compréhension de l’infection à P. hominis.
Key words: Pentatrichomonas hominis / IPEC-J2 / cell viability / inflammatory response
© Y. Zhu et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Trichomonads are ancient eukaryotic protists that inhabit warm, moist, and anaerobic mucosal environments within vertebrate and invertebrate hosts [7]. While numerous studies have extensively investigated trichomonads, particularly focusing on Trichomonas vaginalis and its implications in the reproductive tract [27] (the causative agent of the most common nonviral sexually transmitted disease, trichomoniasis), mechanistic studies on trichomonads that infect the gastrointestinal tract are scarce. One notable pathogenic species in this context is Pentatrichomonas hominis, which causes gastrointestinal symptoms in both humans and animals. It has been isolated from the reproductive and intestinal tracts of bovines [25]. To understand the significance of these infections and develop effective treatment strategies, it is imperative to investigate the virulence factors and pathogenesis of diseases caused by trichomonads that infect the gastrointestinal tract.
Pentatrichomonas hominis is an anaerobic flagellated protozoan that primarily colonizes the large intestines of mammals and is primarily transmitted through the fecal-oral route [20]. Initially considered a commensal protozoan, P. hominis has been found to induce gastrointestinal symptoms, including diarrhea, in humans [1], dogs [24], and cats [3]. It has also been linked to systemic lupus erythematosus [23], irritable bowel syndrome [30], and rheumatoid arthritis [6] in humans. Notably, a significant number of P. hominis infections have been reported in Chinese patients with gastrointestinal cancer [36]. Given its zoonotic potential and pathogenic nature, numerous studies have been conducted to investigate its prevalence and pathogenicity in various vertebrates, including dogs, cattle, pigs, monkeys, and other animals. However, our understanding of P. hominis pathogenicity in animals, particularly in pigs, remains limited. Considering the economic importance of pigs in the food production industry and the potential risk of zoonotic transmission, it is essential to investigate the pathogenic potential of P. hominis in pigs for disease prevention, control strategies, and assessing the risk of transmission to humans.
In recent years, several studies have focused on developing in vitro infection models for trichomonads [18, 19, 31], to investigate their colonization and pathogenic mechanisms. These models serve as valuable tools for examining the establishment of trichomonads and the harm caused by them. The co-cultivation of trichomonads with intestinal porcine epithelial cells (IPEC-J2 cells), originally developed by the Gookin group using T. foetus (which also infects pigs) [33], served as an important foundation for our study. Pentatrichomonas hominis has been detected in the feces of piglets with diarrhea, posing a potential risk to both animal and public health. However, research on the dynamics of infection and pathogenicity of P. hominis, particularly within the epithelial cell system of the pig digestive tract, is limited. Therefore, this study aimed to establish an infection model for the PHGD strain of P. hominis in IPEC-J2 cells. The findings of this study will provide valuable insights into the epidemiology and control of P. hominis in pig populations.
Materials and methods
Parasite isolation and culture
The P. hominis PHGD strain used in this study was isolated from the feces of diarrheal piglets and preserved in the laboratory. The steps involved in this process are as follows: fresh fecal samples were collected and processed within 2 h of collection. The samples were diluted and washed in phosphate-buffered saline (PBS) to remove large particles. The filtrate was centrifuged at 500 × g for 10 min to concentrate the parasites and obtain pellets. Subsequently, the pellet was resuspended in a complete culture medium composed of Modified Diamond’s medium [12], supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL). The parasites were subsequently cultured in 25 cm2 cell culture flasks at 37 °C under anoxic conditions.
IPEC-J2 cell culture
Porcine IPEC-J2 intestinal cells were obtained from a commercial source and cultured following standard protocols [4]. The cells were cultured in a complete culture medium, consisting of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) (Gibco, USA) supplemented with 10% FBS (Gibco, USA) and 1% penicillin-streptomycin (100 U/mL). The cells were maintained at 37 °C with 5% CO2, and the culture medium was refreshed every 2–3 day. Subculturing was performed when the cells reached approximately 80% confluence.
Co-cultivation of P. hominis with IPEC-J2 Cells
IPEC-J2 cells were seeded in 24-well cell culture plates at a density of 2.5 × 105 cells/well and allowed to grow for 24 h to reach approximately 80% confluence. The P. hominis PHGD strain was harvested from the culture flask and resuspended in a complete culture medium. Different concentrations of parasites were introduced into the wells containing IPEC-J2 cells, including (1) a control group without parasites and (2) varying concentrations of parasites (e.g., 104 cells/mL, 105 cells/mL, and 106 cells/mL). The plates were then incubated at 37 °C with 5% CO2 for the desired co-cultivation duration.
Evaluation of P. hominis growth
To assess the growth of P. hominis, the parasite count was monitored at various time points during co-cultivation. Different concentrations of P. hominis PHGD were introduced to each group, including 104 cells/mL, 105 cells/mL, 106 cells/mL, and a control group containing 10% FBS-supplemented DMEM/F-12 medium without IPEC-J2 cells. Following co-cultivation for specific durations of 12, 24, 36, 48, 60, and 72 h, the cells were treated with a 0.25% trypsin solution to release the parasites, and all parasites within each well were collected for enumeration. The collected samples were then centrifuged to concentrate the parasites, which were then resuspended in PBS. Enumeration of the parasites was performed using either a hemocytometer or an automated cell counter. To ensure the reliability and reproducibility of the results, each experimental condition was replicated three times.
Morphological observations
The adhesion of P. hominis to IPEC-J2 cells was assessed through an adhesion assay. The cells were exposed to varying concentrations of P. hominis PHGD, including a control group treated with 1 mL of 10% FBS-supplemented DMEM/F-12 medium, alongside concentrations of 104 cells/mL, 105 cells/mL, 106 cells/mL, 5 × 106 cells/mL, and 107 cells/mL. The co-cultivation period lasted for 6, 12, 18, and 24 h, during which the morphological characteristics of the cells were observed by using Leica DMi8 inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) and documented through photography.
Adhesion assay
The adhesion rate of the P. hominis PHGD strain to IPEC-J2 cells was comprehensively determined in this study. The cells were exposed to varying concentrations of P. hominis PHGD, including a control group treated with 1 mL of 10% FBS-supplemented DMEM/F-12 medium, as well as concentrations of 104 cells/mL, 105 cells/mL, and 106 cells/mL. Following co-cultivation for specific time intervals of 12, 24, 36, 48, 60, and 72 h, the complete culture medium containing non-adherent P. hominis was collected from each well (referred to as P1). Subsequently, the cells were digested using a 0.25% trypsin solution (Gibco, Waltham, MA, USA), and the entire digestion solution containing adherent P. hominis was collected from each well (referred to as P2). The number of parasites in each sample was quantified. The adhesion rate was then calculated using Equation (1), as follows:
Cell viability assessment
After co-cultivation with P. hominis, the viability of IPEC-J2 cells was evaluated using a cell viability assay. The cells were exposed to varying concentrations of P. hominis PHGD, encompassing a control group treated with 100 μL 10% FBS-supplemented DMEM/F-12 medium, as well as concentrations of 104 cells/mL, 105 cells/mL, 106 cells/mL, 5 × 106 cells/mL, and 107 cells/mL. Following co-cultivation for 12, 18, and 24 h, the cells were thoroughly washed with sterile PBS to eliminate any adherent P. hominis. Subsequently, a Cell Counting Kit-8 (CCK-8) assay (Beyotime, Haimen, China) was performed in accordance with the manufacturer’s instructions, utilizing Varioskan LUX multimode microplate reader, with absorbance measured at 450 nm to assess cell viability. Each experiment was conducted in triplicate to ensure the reproducibility and reliability of the results.
Gene expression analysis
The study aimed to investigate the regulatory effects of P. hominis PHGD on gene expression in IPEC-J2 cells by comparing two conditions: a control group treated with 1 mL 10% FBS-supplemented DMEM/F-12 medium and a treatment group exposed to a concentration of 106 cells/mL. After an 18 h co-cultivation period, the cells underwent three washes with sterile PBS to eliminate any adherent P. hominis. Subsequently, RNA was extracted from the co-cultured cells using an EZNA Total RNA Kit I (Omega, Norcross, GA, USA), according to the manufacturer’s instructions. The extracted RNA was reverse transcribed into cDNA using an ABScript III RT Master Mix kit (ABclonal, Woburn, MA, USA). Quantitative PCR (qPCR) was performed to analyze the expression levels of target genes listed in Table 1. Figure S1 presents single melting curves, affirming the specificity of PCR products for all analyzed genes. The relative gene expression levels were calculated using the 2−ΔΔCT method, employing β-actin as the reference genes. For qPCR analysis, the TB Green Premix Ex Taq II qPCR kit (Takara Bio Inc., Kusatsu, Japan) was employed, and fluorescence quantification was conducted using a Bio-Rad CFX Connect thermocycler (Bio-Rad, Irvine, CA, USA).
Primers used for quantitative PCR in this study.
Statistical analysis
All data obtained in this study were subjected to statistical analysis using IBM SPSS Statistics 19 software (IBM Corp., Armonk, NY, USA). Parametric data were analyzed using Student’s t-test or one-way analysis of variance (ANOVA), followed by a post hoc Holm-Sidak test for multiple comparisons. Nonparametric data were analyzed using the Mann–Whitney rank-sum test or Kruskal–Wallis ANOVA on ranks. The results are presented as means ± standard deviations (SD). A significance level of p < 0.05 was deemed statistically significant for all analyses. These statistical procedures were employed to ensure robust and reliable interpretation of the data, adhering to established scientific standards for significance testing.
Results
Growth curves of P. hominis PHGD strain in co-cultivation with IPEC-J2 cells
The co-cultivation of P. hominis PHGD strain with IPEC-J2 cells resulted in a notable enhancement in parasite growth. Figure 1 illustrates the growth curves of P. hominis at varying concentrations. In the co-cultivation group, the parasite exhibited exponential growth, reaching its peak at 48 h. In contrast, when cultured in isolation in 10% FBS-supplemented DMEM/F-12 medium, the parasite failed to exhibit any growth, and all parasites succumbed by the 48-h mark. The growth curves displayed variance depending on the initial inoculation concentration of P. hominis PHGD. In the co-cultivation group with an initial inoculation of 104 cells/mL, the parasite demonstrated exponential growth in the first 48 h, followed by a decline. The peak parasite density reached approximately 4.8 × 105 cells/mL at 48 h. For the co-cultivation group with an initial inoculation of 105 cells/mL, the parasite density increased, reaching a peak of approximately 1.2 × 106 cells/mL at 48 h.
 |
Figure 1 Growth curve of P. hominis co-incubated with IPEC-J2 cells. (A) depicts the growth curve with an initial inoculation concentration of 104 cells/mL of P. hominis, while (B) illustrates the response to an initial inoculation of 105 cells/mL of P. hominis. (C) shows the growth curve following an initial inoculation concentration of 106 cells/mL of P. hominis. The y-axis represents the parasite count, and the x-axis denotes the incubation time in hours. The gray squares represent the growth curve results for individual P. hominis, while the black spheres depict the growth curve results when co-incubated with IPEC-J2 cells. |
Adhesion rate of the P. hominis PHGD strain to IPEC-J2 cells
Following the co-incubation of PHGD with IPEC-J2 cells, the adhesion of PHGD to IPEC-J2 cells was assessed, and the results are presented in Figure 2. The adhesion rate of PHGD to IPEC-J2 cells gradually decreased with the extension of co-incubation time and the increase of the initial inoculation amount. At 12 and 24 h, with an initial inoculation amount of 104 cells/mL and 105 cells/mL, the adhesion rate of PHGD to IPEC-J2 cells was significantly higher compared to other time points. The adhesion rate reached a maximum of 93.82% and 86.57% at 24 h following co-incubation for these two inoculation amounts, respectively. When the initial inoculation amount was 106 cells/mL and incubated for 12 h, the adhesion rate of PHGD to IPEC-J2 cells was approximately 74.52%, which was significantly higher than that at other time points with the same inoculation concentration. At 12, 36, 48, 60, and 72 h, the adhesion rate of PHGD with an initial inoculation amount of 104 cells/mL to IPEC-J2 cells was significantly higher than that at 105 cells/mL and 106 cells/mL.
 |
Figure 2 Adhesion rate of IPEC-J2 cells co-incubated with varying P. hominis concentrations. The adhesion rate is depicted as a bar graph, with the y-axis indicating the percentage of adhered cells, and the x-axis representing the incubation time in hours. Different colors and patterns represent the three initial inoculation amounts. To assess statistical significance, a one-way analysis of variance (ANOVA) was conducted. Letters A, B, and C indicate differences in initial inoculation amounts at the same time point, while lowercase letters a, b, c, d, e, and f denote differences in time points for the same initial inoculation amount. Similar letters indicate no significant difference. |
Morphological changes in IPEC-J2 cells induced by the P. hominis PHGD strain
Co-cultivation with the P. hominis PHGD strain resulted in morphological changes in IPEC-J2 cells, as shown in Figure 3. Compared to the control group, the cells co-cultivated with P. hominis exhibited elongated and irregular shapes. The extent of these morphological changes increased with higher concentrations of P. hominis. At 24 h of co-cultivation, the cells in the control group maintained a typical cobblestone-like morphology, while the cells co-cultivated with P. hominis displayed elongated and stretched shapes. When IPEC-J2 cells were initially inoculated with 1 × 106 cells/mL of P. hominis, significant proliferation of P. hominis was observed after 6 h of co-incubation, and the fusion of IPEC-J2 cells decreased after 18 h. In the case of initial inoculations of 5 × 106 cells/mL and 1 × 107 cells/mL of P. hominis, the high density of parasites after 6 h of co-incubation resulted in a significant number of cell deaths. These morphological changes indicate that the P. hominis PHGD strain can indeed impact the morphology of IPEC-J2 cells.
 |
Figure 3 Morphological changes of IPEC-J2 cells after incubation with different concentrations of P. hominis. Microscopic images (200×) illustrating morphological alterations in IPEC-J2 cells co-incubated with varying concentrations (1 × 104 cells/mL, 1 × 105 cells/mL, 1 × 106 cells/mL, 5 × 106 cells/mL, 1 × 107 cells/mL) of P. hominis (2.5 × 105 IPEC-J2 cells) at different time points (6 h, 12 h, 18 h and 24 h). The black arrow marks the partially visible P. hominis parasites. |
Viability of IPEC-J2 cells following co-cultivation with the P. hominis PHGD strain
The viability of IPEC-J2 cells was evaluated following co-cultivation with the P. hominis PHGD strain at varying concentrations and time points. The results, as depicted in Figure 4, revealed a decrease in cell viability with an increase in the concentration of P. hominis and extension of the co-cultivation period. Specifically, when IPEC-J2 cells were initially inoculated with 5 × 106 cells/mL and 1 × 107 cells/mL of P. hominis, a substantial reduction in the viability of IPEC-J2 cells was observed after 12, 18, and 24 h of co-incubation, compared with the control group. However, when the initial inoculation concentration was 1 × 106 cells/mL of P. hominis, a significant decrease in the viability of IPEC-J2 cells was only observable after 18 h of co-incubation. These findings suggested that the P. hominis PHGD strain exerts an influence on the viability of IPEC-J2 cells in a concentration and time-dependent manner.
 |
Figure 4 Determination of cell viability of IPEC-J2 cells induced by varying concentrations of P. hominis. Cell viability was monitored by using CCK8 assay kits, in which optical density (OD) 450 nm values represent cell viability. The y-axis denotes the OD450 nm, and the x-axis indicates the various P. hominis co-culture time points. The data points are represented as a line graph, illustrating the trend of cell viability across the concentration range. Statistical significance is denoted by asterisks: *p < 0.05, **p < 0.001, and ***p < 0.0001. |
Changes in mRNA expression levels of related genes in IPEC-J2 cells induced by the P. hominis PHGD strain
The mRNA expression levels of various genes associated with cell damage and inflammation in IPEC-J2 cells were examined following co-cultivation with the P. hominis PHGD strain (Figure 5 and Table S1). The results revealed significant upregulation in the expression levels of IL-6, IL-8, and TNF-α within the co-cultivation group compared to the control group (p < 0.05). Conversely, the expression levels of CAT and CuZn-SOD experienced significant downregulation (p < 0.05). These genes play pivotal roles in inflammatory responses, apoptosis, and cell survival. The upregulation of inflammatory genes and the concurrent downregulation of anti-oxidative stress genes indicate that the P. hominis PHGD strain can trigger inflammation within IPEC-J2 cells. These findings provide valuable insights into the mechanisms through which P. hominis may potentially induce cellular damage and trigger inflammatory responses in the context of IPEC-J2 cell co-cultivation.
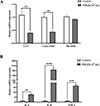 |
Figure 5 Alterations in mRNA expression in IPEC-J2 cells induced by P. hominis. This figure illustrates the alterations in mRNA expression of specific genes in IPEC-J2 cells following P. hominis induction. (A) displays changes in mRNA expression of anti-oxidative stress-related genes, while (B) presents changes in mRNA expression of inflammatory-related genes. The significance of the differences between the control group and the P. hominis-induced group is indicated by asterisks: *p < 0.05, **p < 0.001, and ***p < 0.0001. |
Discussion
Pentatrichomonas hominis is a parasitic protozoan known to be endemic in various regions and displays a lack of host specificity. It primarily parasitizes the digestive tracts of its hosts. The pathogenicity of P. hominis has long been a subject of debate. In the past, it was considered a symbiotic or conditionally pathogenic entity within the intestines of both humans and animals. However, recent studies have reported the presence of P. hominis in the feces of affected individuals and animals [2, 3, 6, 24].
One pivotal study conducted by Chudnovskiy et al. [5] demonstrated that Tritrichomonas musculis, a newfound member of the Trichomonadaceae, contributes to the development of enteritis and colon cancer. Notably, a high infection rate of P. hominis (41.54%) was reported in patients with gastrointestinal cancer in China, significantly surpassing the 9.15% infection rate observed in the general population [36]. A more in-depth analysis of the gut microbiota in colon cancer patients with and without P. hominis infection, utilizing 16S rRNA high-throughput sequencing, revealed a higher abundance of colon cancer-related bacteria in P. hominis-infected individuals [35]. Despite these associations, the causal relationship between P. hominis infection and gastrointestinal cancer remains uncertain, necessitating further research to establish a potential causal link.
Moreover, P. hominis has also been identified as the causative agent of diarrhea in dogs and cats, which are often companions to humans [3, 24, 26]. Consequently, the study of P. hominis pathogenicity has gained increasing attention. As extracellular parasitic pathogens, trichomonads such as P. hominis can adhere to epithelial cells, interact with the host immune system, and influence the microbiota composition. Fang et al. [8] significantly contributed to our understanding of P. hominis in response to a zoonotic emergency through multi-omics research, characterizing hydrogenosomal proteins using RNA sequencing and proteomics techniques. Handrich et al. [16] employed RNA-Seq technology to confirm that the BspA and Pmp gene families are associated with enhanced adhesion of trichomonads to host cells, including P. hominis. Experimental evidence has shown that one T. vaginalis TvBspA-like and two TvPmp-like proteins promoted interactions between T. vaginalis and vaginal epithelial cells. Additionally, Bailey and Hirt [2] emphasized the interplay between trichomonads and the mucosal bacteria, highlighting its impact on overall health. These parasitic entities engage in competition with the intestinal microflora for adhesion with the host’s epithelial cells. Once these connections are established, the parasites can effectively evade host immune response, leading to successful colonization and subsequent infection, potentially resulting in host disease [9].
Our findings revealed that IPEC-J2 cells can facilitate the growth and proliferation of P. hominis in vitro following the interaction between P. hominis and IPEC-J2 cells. Furthermore, P. hominis displayed a propensity to adhere to cells and induce a degree of cellular damage. Previous studies have demonstrated that the d growth of T. vaginalis is enhanced in the presence of eukaryotic cells, with cell culture surpassing clinical broth culture [13]. Upon co-incubation, P. hominis exhibited adherence to IPEC-J2 cells. Intriguingly, an increase in parasite density resulted in a decrease in the adhesion rate, suggesting the presence of competitive adhesion dynamics among the parasites. With extended co-incubation time, the activity and number of IPEC-J2 cells decreased, resulting in a decrease in the adhesion rate of P. hominis to these cells. Notably, trichomonads can modulate the expression of its proteins after adhering to host cells, allowing it to adapt to the current living conditions. This phenomenon also impacts the virulence of trichomonads. Martínez-Herrero et al.’s research found that virulent strains tend to possess a greater abundance of membrane proteins and cell adhesion-related proteins, whereas the proteins of attenuated strains are predominantly associated with carbohydrate metabolism [29].
The intricate network of parasite-microbiome-host interactions involving trichomonads is characterized by their adherence to host cells, leading to severe pathological manifestations. These interactions, as observed in the case of P. hominis, result in mucosal ecological imbalances and, consequently, potentially benefit the trichomonads themselves [17]. Similar to previous studies on T. vaginalis and T. foetus [34], P. hominis has also demonstrated the ability to adhere to target cells and induce significant monolayer contraction. Trichomonad adhesion has the potential to induce alterations in mitochondrial membrane potential, resulting in substantial cytotoxicity. The cytotoxicity levels induced by different strains of trichomonads vary among clinical isolates, indicating that the ability to adhere and damage host cells is strain-dependent [21]. Our results revealed that P. hominis can adhere to host cells and induce a certain degree of cellular damage. However, there is no strict linear correlation between the adhesion capability and the damage potential of P. hominis for host cells. It should be emphasized that only when a sufficient number of P. hominis adhere to cells can they inflict cellular damage. This supports the notion that adhesion alone is not sufficient to cause cell damage, and it is more likely attributed to different specific effectors among the strains [28].
Our exploration of mRNA expression levels related to inflammation in IPEC-J2 cells, specifically the increased expression of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) in response to P. hominis, is placed within a broader context illuminated by relevant research. Hong et al. [18] investigated the induction of IL-6 production by T. tenax on oral and pulmonary epithelial cells, revealing parallels with our observations of P. hominis-induced inflammation. Additionally, Han et al. [15] delved into signaling pathways associated with IL-6 production and epithelial-mesenchymal transition in prostate epithelial cells stimulated by T. vaginalis, providing insights into the molecular mechanisms underlying inflammation during microbial infections. Moreover, studies have highlighted the contribution of the vaginal microbiota to trichomonad-induced inflammatory responses. Fiori et al. [11] demonstrated that the presence of Mycoplasma hominis in T. vaginalis isolates synergistically upregulates proinflammatory cytokines, aligning with our findings. Fichorova et al. [10] explored intricate interactions among vaginal microorganisms, emphasizing the collective impact of T. vaginalis and bacterial vaginosis on inflammation and adverse reproductive outcomes. Collectively, these studies substantiate our investigation into the interplay between microbial infections, inflammation, and immune regulation, underscoring the broader significance of comprehending host-pathogen interactions across diverse cellular contexts. Subsequent research could further delve into the regulatory roles of the gastrointestinal microbiota and P. hominis in modulating inflammatory responses.
The generation of reactive oxygen species (ROS) is a critical factor in inducing apoptosis in mammalian cells following infection with various pathogens [14]. Quan et al. [32] reported that T. vaginalis induces mitochondrial ROS production in SiHa cells through adhesion and infection in a time-dependent manner, thereby promoting apoptosis. In our study, the expression of anti-oxidative stress-related genes, such as CAT and CuZn-SOD, was significantly down-regulated following co-incubation of P. hominis with IPEC-J2 cells. This observation further confirms that P. hominis can induce certain damage in IPEC-J2 cells.
In conclusion, our study confirms that P. hominis can adhere to and rely on IPEC-J2 cells for growth and proliferation. Additionally, P. hominis can affect IPEC-J2 cells to varying degrees, leading to alterations in cell morphology and reductions in cell viability. The co-incubation of IPEC-J2 cells with P. hominis resulted in the down-regulation of anti-oxidative stress-related genes and the up-regulation of inflammatory-related genes. Therefore, we assert that P. hominis infection can damage intestinal epithelial cells and trigger associated inflammatory responses. These findings provide evidence for the pathogenic potential of P. hominis and serve as a baseline for further exploration of its pathogenesis. Further in vitro and in vivo studies are needed to validate our findings and may potentially reshape the current understanding of P. hominis infection.
Funding
The National Key R&D Program of China (2023YFD1801205) open competition program on the top 10 critical priorities of the Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2023SDZG02), Science and Technology Plan Projects of Guangdong Province (2021B1212050021), Guangdong Basic and Applied Basic Research Foundation (2021B1515120006), Opening Project of State Key Laboratory of Swine and Poultry Breeding Industry (2023QZ-NK05), Science and technology project of Guangzhou (2023B04J0137, 2023A04J0789), The project of Key Laboratory of Livestock Disease Prevention of Guangdong Province (2023B1212060040), special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (202110TD, 202122TD, R2020PY-JC001, R2019YJ-YB3010, R2020PY-JG013, R2020QD-048, R2021PY-QY007, R2023PY-JG018), Guangdong Provincial special fund for modern Agriculture Industry Technology Innovation teams (2022KJ119), and the Project of Collaborative Innovation Center of GDAAS (XTXM202202).
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
Y.Z.: Conceptualization, Data Curation. H.C.: Data Curation, Investigation. S.F., J.L.: Investigation. N.Q.: Investigation. Z.Y., M.L.: Data Curation. H.S., X.L.: Investigation. Y.S.: Investigation. J.H.: Investigation. J.Z.: Investigation. X.C.: Investigation. D.W., L.Y.: Investigation. S.L.: Project Administration, Resources, Writing – Review and Editing. M.S.: Conceptualization, Funding Acquisition, Project Administration, Resources, Writing – Review and Editing. All authors have read and agreed to the published version of the manuscript.
Supplementary material
 |
Figure S1: Melt curves of the target genes: CAT (A), CuZn-SOD (B), Mn-SOD (C), IL-6 (D), IL-8 (E), TNF-α (F), and β-actin (G). |
Table S1: Comprehensive QPCR results for alterations in mRNA expression in IPEC-J2 cells induced by P. hominis. Access here
References
- Abdo SM, Ghallab MMI, Elhawary NM, Elhadad H. 2022. Pentatrichomonas hominis and other intestinal parasites in school-aged children: Coproscopic survey. Journal of Parasitic Diseases, 46, 896–900. [CrossRef] [PubMed] [Google Scholar]
- Bailey NP, Hirt RP. 2023. Revisiting fecal metatranscriptomics analyses of macaques with idiopathic chronic diarrhoea with a focus on trichomonad parasites. Parasitology, 150, 248–261. [CrossRef] [PubMed] [Google Scholar]
- Bastos BF, Brener B, de Figueiredo MA, Leles D, Mendes-de-Almeida F. 2018. Pentatrichomonas hominis infection in two domestic cats with chronic diarrhea. Journal of Feline Medicine and Surgery Open Reports, 4, 1–4. [Google Scholar]
- Chen Q, Yu M, Tian Z, Cui Y, Deng D, Rong T, Liu Z, Song M, Li Z, Ma X, Lu H. 2022. Exogenous glutathione protects IPEC-J2 cells against oxidative stress through a mitochondrial mechanism. Molecules, 27, 2416. [CrossRef] [PubMed] [Google Scholar]
- Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, Amir ED, Solovyov A, Greenbaum B, Clemente J, Faith J, Belkaid Y, Grigg ME, Merad M. 2016. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell, 167, 444–456.e14. [CrossRef] [PubMed] [Google Scholar]
- Compaoré C, Lekpa FK, Nebie L, Niamba P, Niakara A. 2013. Pentatrichomonas hominis infection in rheumatoid arthritis treated with adalimumab. Rheumatology, 52, 1534–1535. [CrossRef] [PubMed] [Google Scholar]
- Dimasuay KGB, Rivera WL. 2013. Molecular characterization of trichomonads isolated from animal hosts in the Philippines. Veterinary Parasitology, 196, 289–295. [CrossRef] [PubMed] [Google Scholar]
- Fang YK, Chien KY, Huang KY, Cheng WH, Ku FM, Lin R, Chen TW, Huang PJ, Chiu CH, Tang P. 2016. Responding to a zoonotic emergency with multi-omics research: Pentatrichomonas hominis hydrogenosomal protein characterization with use of RNA sequencing and proteomics. OMICS A Journal of Integrative Biology, 20, 662–669. [CrossRef] [PubMed] [Google Scholar]
- Fichorova R, Fraga J, Rappelli P, Fiori PL. 2017. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Research in Microbiology, 168, 882–891. [CrossRef] [PubMed] [Google Scholar]
- Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB. 2013. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sexually Transmitted Infections, 89, 460–466. [CrossRef] [PubMed] [Google Scholar]
- Fiori PL, Diaz N, Cocco AR, Rappelli P, Dessi D. 2013. Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sexually Transmitted Infections, 89, 449–454. [CrossRef] [PubMed] [Google Scholar]
- Fouts AC, Kraus SJ. 1980. Trichomonas vaginalis: Reevaluation of its clinical presentation and laboratory diagnosis. Journal of Infectious Diseases, 141, 137–143. [CrossRef] [PubMed] [Google Scholar]
- Garber GE, Sibau L, Ma R, Proctor EM, Shaw CE, Bowie WR. 1987. Cell culture compared with broth for detection of Trichomonas vaginalis. Journal of Clinical Microbiology, 25, 1275–1279. [CrossRef] [PubMed] [Google Scholar]
- Go D, Lee J, Choi JA, Cho SN, Kim SH, Son SH, Song CH. 2019. Reactive oxygen species-mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium-infected macrophages by activating regulated IRE1-dependent decay pathway. Cellular Microbiology, 21, e13094. [PubMed] [Google Scholar]
- Han IH, Kim JH, Kim SS, Ahn MH, Ryu JS. 2016. Signalling pathways associated with IL-6 production and epithelial–mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunology, 38, 678–687. [CrossRef] [PubMed] [Google Scholar]
- Handrich MR, Garg SG, Sommerville EW, Hirt RP, Gould SB. 2019. Characterization of the BspA and Pmp protein family of trichomonads. Parasites & Vectors, 12, 406. [CrossRef] [PubMed] [Google Scholar]
- Hirt RP. 2019. Mucosal microbial parasites/symbionts in health and disease: an integrative overview. Parasitology, 146, 1109–1115. [CrossRef] [PubMed] [Google Scholar]
- Hong Z-B, Lai Y-T, Chen C-H, Chen Y-J, Chen C-C, Lin W-C. 2023. Trichomonas tenax induces barrier defects and modulates the inflammatory cytotoxicity of gingival and pulmonary epithelial cells. Parasite, 30, 7. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Iriarte LS, Martinez CI, de Miguel N, Coceres VM. 2023. Tritrichomonas foetus cell division involves dna endoreplication and multiple fissions. Microbiology Spectrum, 11, e0325122. [CrossRef] [PubMed] [Google Scholar]
- Itoh N, Iijima Y, Ogura I, Yonekura N, Kameshima S, Kimura Y. 2020. Molecular prevalence of trichomonad species from pet shop puppies and kittens in Japan. Revista Brasileira de Parasitologia Veterinaria, 29, 1–6. [Google Scholar]
- Jesus JB, Vannier-Santos MA, Britto C, Godefroy P, Silva-Filho FC, Pinheiro AAS, Rocha-Azevedo B, Lopes AHCS, Meyer-Fernandes JR. 2004. Trichomonas vaginalis virulence against epithelial cells and morphological variability: The comparison between a well-established strain and a fresh isolate. Parasitology Research, 93, 369–377. [CrossRef] [PubMed] [Google Scholar]
- Ji X, Zheng W, Yao W. 2019. Protective role of hydrogen gas on oxidative damage and apoptosis in intestinal porcine epithelial cells (IPEC-J2) induced by deoxynivalenol: A preliminary study. Toxins, 12, 5. [Google Scholar]
- Jongwutiwes S, Silachamroon U, Putaporntip C. 2000. Pentatrichomonas hominis in empyema thoracis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 94, 185–186. [CrossRef] [PubMed] [Google Scholar]
- Li WC, Gong PT, Ying M, Li JH, Yang J, Li H, Yang ZT, Zhang GC, Zhang XC. 2014. Pentatrichomonas hominis: First isolation from the feces of a dog with diarrhea in China. Parasitology Research, 113, 1795–1801. [CrossRef] [PubMed] [Google Scholar]
- Li WC, Huang JM, Fang Z, Ren Q, Tang L, Kan ZZ, Liu XC, Gu YF. 2020. Prevalence of Tetratrichomonas buttreyi and Pentatrichomonas hominis in yellow cattle, dairy cattle, and water buffalo in China. Parasitology Research, 119, 637–647. [CrossRef] [PubMed] [Google Scholar]
- Li WC, Ying M, Gong PT, Li JH, Yang J, Li H, Zhang XC. 2016. Pentatrichomonas hominis: prevalence and molecular characterization in humans, dogs, and monkeys in Northern China. Parasitology Research, 115, 569–574. [CrossRef] [PubMed] [Google Scholar]
- Liu J, Zeng M, Yang L, Mao Y, He Y, Li M, Chen Q, Zhou W, Chen L, Zhu Q. 2022. Prevalence of reproductive tract infections among women preparing to conceive in Chongqing, China: Trends and risk factors. Reproductive Health, 19, 1–9. [CrossRef] [PubMed] [Google Scholar]
- Lustig G, Ryan CM, Secor WE, Johnson PJ. 2013. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infection and Immunity, 81, 1411–1419. [CrossRef] [PubMed] [Google Scholar]
- Martínez-Herrero MDC, Garijo-Toledo MM, González F, Bilic I, Liebhart D, Ganas P, Hess M, Gómez-Muñoz MT. 2019. Membrane associated proteins of two Trichomonas gallinae clones vary with the virulence. PLoS One, 14, e0224032. [CrossRef] [PubMed] [Google Scholar]
- Meloni D, Mantini C, Goustille J, Desoubeaux G, Maakaroun-Vermesse Z, Chandenier J, Gantois N, Duboucher C, Fiori PL, Dei-Cas E, Duong TH, Viscogliosi E. 2011. Molecular identification of Pentatrichomonas hominis in two patients with gastrointestinal symptoms. Journal of Clinical Pathology, 64, 933–935. [CrossRef] [PubMed] [Google Scholar]
- Miranda-Ozuna JFT, Rivera-Rivas LA, Cárdenas-Guerra RE, Hernández-García MS, Rodríguez-Cruz S, González-Robles A, Chavez-Munguía B, Arroyo R. 2019. Glucose-restriction increases Trichomonas vaginalis cellular damage towards HeLa cells and proteolytic activity of cysteine proteinases (CPs), such as TvCP2. Parasitology, 146, 1156–1166. [CrossRef] [PubMed] [Google Scholar]
- Quan JH, Kang BH, Cha GH, Zhou W, Koh YB, Yang JB, Yoo HJ, Lee MA, Ryu JS, Noh HT, Kwon J, Lee YH. 2014. Trichonomas vaginalis metalloproteinase induces apoptosis of SiHa cells through disrupting the Mcl-1/ Bim and Bcl-xL/Bim complexes. PLoS ONE, 9, e110659. [CrossRef] [PubMed] [Google Scholar]
- Tolbert MK, Stauffer SH, Gookin JL. 2013. Feline Tritrichomonas foetus adhere to intestinal epithelium by receptor-ligand-dependent mechanisms. Veterinary Parasitology, 192, 75–82. [CrossRef] [PubMed] [Google Scholar]
- Vilela RC, Benchimol M. 2012. Trichomonas vaginalis and tritrichomonas foetus: Interaction with fibroblasts and muscle cells – new insights into parasite-mediated host cell cytotoxicity. Memórias do Instituto Oswaldo Cruz, 107, 720–727. [CrossRef] [PubMed] [Google Scholar]
- Zhang H, Yu Y, Li J, Gong P, Wang X, Li X, Cheng Y, Yu X, Zhang N, Zhang X. 2022. Changes of gut microbiota in colorectal cancer patients with Pentatrichomonas hominis infection. Frontiers in Cellular and Infection Microbiology, 12, 961974. [CrossRef] [PubMed] [Google Scholar]
- Zhang N, Zhang H, Yu Y, Gong P, Li J, Li Z, Li T, Cong Z, Tian C, Liu X, Yu X, Zhang X. 2019. High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasites & Vectors, 12, 423. [CrossRef] [PubMed] [Google Scholar]
- Zhou Y, Wang B, Wang Q, Tang L, Zou P, Zeng Z, Zhang H, Gong L, Li W. 2021. Protective effects of Lactobacillus plantarum Lac16 on Clostridium perfringens infection-associated injury in IPEC-J2 cells. International Journal of Molecular Sciences, 22, 12388. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Zhu Y, Cai H, Fang S, Shen H, Yan Z, Wang D, Qi N, Li J, Lv M, Lin X, Hu J, Song Y, Chen X, Yin L, Zhang J, Liao S & Sun M. 2024. Unraveling the pathogenic potential of the Pentatrichomonas hominis PHGD strain: impact on IPEC-J2 cell growth, adhesion, and gene expression. Parasite 31, 18.
All Tables
All Figures
 |
Figure 1 Growth curve of P. hominis co-incubated with IPEC-J2 cells. (A) depicts the growth curve with an initial inoculation concentration of 104 cells/mL of P. hominis, while (B) illustrates the response to an initial inoculation of 105 cells/mL of P. hominis. (C) shows the growth curve following an initial inoculation concentration of 106 cells/mL of P. hominis. The y-axis represents the parasite count, and the x-axis denotes the incubation time in hours. The gray squares represent the growth curve results for individual P. hominis, while the black spheres depict the growth curve results when co-incubated with IPEC-J2 cells. |
| In the text | |
 |
Figure 2 Adhesion rate of IPEC-J2 cells co-incubated with varying P. hominis concentrations. The adhesion rate is depicted as a bar graph, with the y-axis indicating the percentage of adhered cells, and the x-axis representing the incubation time in hours. Different colors and patterns represent the three initial inoculation amounts. To assess statistical significance, a one-way analysis of variance (ANOVA) was conducted. Letters A, B, and C indicate differences in initial inoculation amounts at the same time point, while lowercase letters a, b, c, d, e, and f denote differences in time points for the same initial inoculation amount. Similar letters indicate no significant difference. |
| In the text | |
 |
Figure 3 Morphological changes of IPEC-J2 cells after incubation with different concentrations of P. hominis. Microscopic images (200×) illustrating morphological alterations in IPEC-J2 cells co-incubated with varying concentrations (1 × 104 cells/mL, 1 × 105 cells/mL, 1 × 106 cells/mL, 5 × 106 cells/mL, 1 × 107 cells/mL) of P. hominis (2.5 × 105 IPEC-J2 cells) at different time points (6 h, 12 h, 18 h and 24 h). The black arrow marks the partially visible P. hominis parasites. |
| In the text | |
 |
Figure 4 Determination of cell viability of IPEC-J2 cells induced by varying concentrations of P. hominis. Cell viability was monitored by using CCK8 assay kits, in which optical density (OD) 450 nm values represent cell viability. The y-axis denotes the OD450 nm, and the x-axis indicates the various P. hominis co-culture time points. The data points are represented as a line graph, illustrating the trend of cell viability across the concentration range. Statistical significance is denoted by asterisks: *p < 0.05, **p < 0.001, and ***p < 0.0001. |
| In the text | |
 |
Figure 5 Alterations in mRNA expression in IPEC-J2 cells induced by P. hominis. This figure illustrates the alterations in mRNA expression of specific genes in IPEC-J2 cells following P. hominis induction. (A) displays changes in mRNA expression of anti-oxidative stress-related genes, while (B) presents changes in mRNA expression of inflammatory-related genes. The significance of the differences between the control group and the P. hominis-induced group is indicated by asterisks: *p < 0.05, **p < 0.001, and ***p < 0.0001. |
| In the text | |
 |
Figure S1: Melt curves of the target genes: CAT (A), CuZn-SOD (B), Mn-SOD (C), IL-6 (D), IL-8 (E), TNF-α (F), and β-actin (G). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.