| Issue |
Parasite
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 50 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/parasite/2025043 | |
| Published online | 05 August 2025 | |
Research Article
Prevalence, morphological and molecular characterization of Leucocytozoon macleani (Apicomplexa: Haemosporida) from chickens in Thailand
Prévalence, caractérisation morphologique et moléculaire de Leucocytozoon macleani (Apicomplexa : Haemosporida) chez les poulets en Thaïlande
1
Department of Animal Science, Faculty of Natural Resources, Rajamangala University of Technology Isan, Sakon Nakhon, 47160, Thailand
2
State Scientific Research Institute Nature Research Centre, Vilnius, 08412, Lithuania
3
Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong Special Administrative Region, PR China
4
Akkhraratchakumari Veterinary College, Walailak University, Nakhon Si Thammarat, 80160, Thailand
5
Informatics Innovation Center of Excellence, Walailak University, Nakhon Si Thammarat, 80160, Thailand
6
One Health Research Center, Walailak University, Nakhon Si Thammarat, 80160, Thailand
* Corresponding author: pp.vettech@gmail.com
Received:
17
April
2025
Accepted:
9
July
2025
Leucocytozoon species are common in countries with warm climates but are an often neglected blood parasite in poultry. Although Leucocytozoon macleani is less virulent than Leucocytozoon caulleryi, it can still negatively impact production performance. In Thailand, the available reports indicate a high prevalence of Leucocytozoon spp., but detailed morphological characteristics of the parasites remain insufficiently known. In this study, Giemsa-stained blood smears and extracted genomic (g) DNA were obtained from 60 domestic chickens (Gallus gallus domesticus). Blood smears were examined for the presence of Leucocytozoon species and their morphological characteristics were examined. A total of 60 gDNA samples were used for nested-PCR amplification of the cytochrome b gene of Leucocytozoon species, followed by sequencing and phylogenetic analysis. The microscopic and molecular examinations revealed prevalence of leucocytozoonosis in chickens of 85% and 90%, respectively. Sequence analysis indicated that several infected chickens harboured multiple Leucocytozoon lineages. Leucocytozoon macleani was morphologically identified in nine samples and could be linked to the lineages GALLUS17, GALLUS34, and the new lineages GALLUS63. The found gametocytes of L. macleani morphologically resembled those reported previously, but exhibited some distinct characteristics. Phylogenetically, the lineages of L. macleani isolated in this study grouped separately from some other L. macleani lineages deposited in GenBank. In conclusion, the prevalence of Leucocytozoon infection in chickens from Northeastern Thailand was high, with frequent co-infections by multiple lineages. Leucocytozoon macleani may exhibit cryptic specification. This study is the first report of L. macleani lineages described using MalAvi database nomenclature, alongside their morphological characteristics.
Résumé
Les espèces de Leucocytozoon sont courantes dans les pays à climat chaud, mais sont souvent négligées comme parasites sanguins chez les volailles. Bien que Leucocytozoon macleani soit moins virulent que Leucocytozoon caulleryi, il peut néanmoins avoir un impact négatif sur les performances de production. En Thaïlande, les rapports disponibles indiquent une prévalence élevée de Leucocytozoon spp., mais les caractéristiques morphologiques détaillées des parasites restent insuffisamment connues. Dans cette étude, des frottis sanguins colorés au Giemsa et de l’ADN génomique (g) extrait ont été obtenus chez 60 poulets domestiques (Gallus gallus domesticus). Les frottis sanguins ont été examinés pour détecter la présence d’espèces de Leucocytozoon et leurs caractéristiques morphologiques ont été étudiées. Soixante échantillons d’ADNg ont été utilisés pour l’amplification par PCR nichée du gène du cytochrome b des espèces de Leucocytozoon, suivie d’un séquençage et d’une analyse phylogénétique. Les examens microscopiques et moléculaires ont révélé respectivement une prévalence de leucocytozoonose chez les poulets de 85% et 90%. L’analyse des séquences a indiqué que plusieurs poulets infectés hébergeaient plusieurs lignées de Leucocytozoon. Leucocytozoon macleani a été identifié morphologiquement dans neuf échantillons et pourrait être lié aux lignées GALLUS17, GALLUS34 et aux nouvelles lignées GALLUS63. Les gamétocytes de L. macleani trouvés ressemblaient morphologiquement à ceux rapportés précédemment, mais présentaient certaines caractéristiques distinctes. Phylogénétiquement, les lignées de L. macleani isolées dans cette étude se sont regroupées séparément de certaines autres lignées de L. macleani déposées dans GenBank. En conclusion, la prévalence de l’infection à Leucocytozoon chez les poulets du nord-est de la Thaïlande était élevée, avec de fréquentes co-infections par plusieurs lignées. Leucocytozoon macleani pourrait présenter une spécification cryptique. Cette étude est le premier rapport sur les lignées de L. macleani décrites à l’aide de la nomenclature de la base de données MalAvi, ainsi que sur leurs caractéristiques morphologiques.
Key words: Blood parasite / Co-infection / Domestic chicken / Leucocytozoon / Morphology / Prevalence
Edited by: Jean-Lou Justine
© Ľ. Juhásová et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Leucocytozoon species (Apicomplexa: Haemosporida) are exclusively bird parasites [64]. They can be distinguished from other haemosporidian parasites, such as Plasmodium, Haemoproteus, by the absence of pigment granules (haemozoin) [63]. Merogony of Leucocytozoon species does not take place in blood cells; only gametocytes are present in blood cells. Leucocytozoon species are heteroxenous parasites, meaning their life cycles involve both birds and insect vectors (blackflies for almost all Leucocytozoon spp. and biting midges for Leucocytozoon caulleryi) [58]. Sexual process and sporogony occur in the insects, while asexual reproduction (merogony or schizogony), and production of gametocytes occur in avian hosts [3]. Infections with Leucocytozoon spp. in birds results in a disease called leucocytozoonosis [59].
About 45 species of Leucocytozoon have been described [58, 64]. Among these, three species – L. caulleryi, L. macleani (syn. L. sabrazesi) and L. schoutedeni (syn. L. andrewsi, L. gallinarum) – are found in domestic chickens (Gallus gallus domesticus) [48]. Leucocytozoon caulleryi is well known for its high virulence, causing fatal haemorrhagic disease in chickens [36]. Haemorrhage may result from the rupture of megalomeronts, which can be developed in many organs, including the pectoral muscle, spleen, liver, intestine, pancreas, kidney, heart, lung and even eyes [11, 50, 56]. In the female reproductive system, large meronts can be found in the ovary and oviduct, leading to inflammation and atrophy in the reproductive tract, and ultimately a drop in egg production and shell-less eggs [30]. Additionally, L. caulleryi-infected laying hens may exhibit anaemia and green diarrhoea [31]. In contrast, L. macleani and L. schoutedeni are less pathogenic, but can still lead to decreased productivity (both egg and meat), anaemia, and green diarrhoea [58].
In Southeast Asia, chicken leucocytozoonosis has been reported in Vietnam, Myanmar, Indonesia, Malaysia, Philippines, Singapore and Thailand [13, 26, 28, 66]. In Thailand, the first case of chicken leucocytozoonosis, known as Bangkok haemorrhagic disease, was reported in 1945 [7, 25]. In recent years, the agents of leucocytozoonosis reported in this region are mainly less virulent species such as L. sabrazesi and Leucocytozoon sp. [5, 25, 35, 53]. Also, cases of pathogenic L. caulleryi have also been found [36], as well as cases of L. schoutedeni, lineages GALLUS06 and GALLUS07, which was molecularly detected from backyard chickens in Thailand [5]. However, identification of Leucocytozoon species is mainly based on the morphological features of their blood stages (gametocytes) and vertebrate-host specificity data [64]. Thus, confirmation of L. schoutedeni infection in Thai chickens requires further investigation, preferably combining microscopic and molecular techniques, which will help to shed light on the transmission of L. schoutedeni in Thailand.
A fragment of 479 bp of the cytochrome b (cytb) gene is widely used as DNA barcoding fragment for Leucocytozoon species detection worldwide. Currently, approximately 1,542 Leucocytozoon lineages are deposited in the MalAvi database (http://130.235.244.92/Malavi/), an online open access database of cytb sequences of haemosporidian parasites [4]. In domestic chickens, there are about 50 described lineages of Leucocytozoon species, primarily from Asia and Africa [4]. Among these, several lineages were not identified to the species level. Additionally, a previous phylogenetic study [5] revealed that some Leucocytozoon resembling L. macleani are grouped separately from L. macleani. This information highlights the high genetic diversity within Leucocytozoon and underscores the challenges in identifying new parasite lineages or previously overlooked species in chickens.
The active transmission of chicken leucocytozoonosis in Thailand suggests that the disease remains a significant concern for poultry health and production. Even though the less pathogenic parasite species were reported, continuous monitoring is needed to prevent their negative impacts [53]. Additionally, information on tissue merogony and damage of internal organs by Leucocytozoon parasites remains fragmentary, but is important for better understanding the mechanisms of pathology during chicken leucocytozoonosis. Takang et al. (2017) suggested that research on Leucocytozoon spp. in Thai chickens is incomplete, and novel lineages and species are potentially overlooked. This gap may hinder a comprehensive understanding of genetic diversity, host-parasite interactions, pathology, and the development of treatment and prevention strategies. Thus, this study aimed to contribute new knowledge to better understand the distribution and identification of Leucocytozoon species and lineages infecting village chickens in Northeastern Thailand, using microscopic and molecular techniques.
Materials and methods
Ethical considerations
All sampling procedures in animals were reviewed and approved by the Walailak University Institutional Animal Care and Use committee (Approval number: WU-ACUC-66091). Handling of samples and molecular analysis of blood parasites were conducted complying with the regulation of the Institutional Biosafety Committee (IBC) of Walailak University (Approval number: WU-IBC-66-066).
Blood collection and sample processing
In March 2024, during the dry season with low rainfall [32], 60 blood samples (0.5 mL) were collected from village chickens (male = 41 and female = 19) raised in Kalasin (n = 27), Roi-Et (n = 18), and Sakon Nakhon (n = 15), Northeastern Thailand (Fig. 1). Fresh blood smears were prepared in triplicate immediately after blood collection. The blood smears were air-dried, fixed in absolute methanol for one minute, and stained with 10% Giemsa solution for one hour [60]. The remaining blood was transferred into an ethylenediaminetetraacetic acid (EDTA) tube (MediPlusTM, Bangkok, Thailand) and transported to the Laboratory of Veterinary Clinical Pathology, Akkhraratchakumari Veterinary College, Walailak University, maintaining cold-chain transportation. Afterwards, the samples were kept at 4 °C until further molecular analysis was performed.
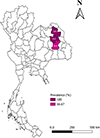 |
Figure 1 Study sites and prevalence of Leucocytozoon species in domestic chickens in Northeastern provinces of Thailand. Kalasin (KLS), Roi-Et (ROE), and Sakon Nakhon (SKN). The figure is generated by QGIS version 3.36.2-Maidenhead. |
Microscopic examination and parasite morphology
Giemsa-stained blood smears were examined for the presence of Leucocytozoon parasites as well as other blood parasites using an Olympus BX43 light microscope (Olympus, Tokyo, Japan) equipped with a digital camera (OlympusDP27, Olympus) and CellSens imaging software (version 1.18, Olympus). The smears were examined at 400× magnification across 100 fields and then re-evaluated at 1000× magnification for 100 fields [60]. Parasitaemia of Leucocytozoon was estimated as a percentage by actual counting of the number of parasites per 10,000 red blood cells [61] and categorised as not infected (no detectable parasites), low (<0.1%), moderate (0.1%–1%), and high (>1%) [65]. Additionally, samples that were PCR-positive for Leucocytozoon sp. were used for morphometric analyses.
Images of macrogametocytes and microgametocytes, captured from samples with parasitaemia ranging from 0.03–0.16%, were analysed using CellSens imaging software (version 1.18, Olympus), following previous protocols [14, 48, 57, 61]. Measurements were conducted on gametocytes of two lineages of L. macleani, GALLUS17 and GALLUS63. For GALLUS17, parasitic forms included both fusiform and roundish macrogametocytes and microgametocytes. In contrast, for GALLUS63, only fusiform macrogametocytes and microgametocytes were included, as roundish forms were rarely observed.
Genomic DNA extraction and nested-PCR
Genomic (g) DNA was extracted from 50 μL of all 60 blood samples, using a Blood Genomic DNA Extraction Mini Kit, following manufacturer’s recommendation (FavorPrep, Pingtung, Taiwan). The initial PCR utilised primers HaemNFI and HaemNR3 [20] to amplify parasite mitochondrial DNA of haemosporidian parasites (Haemoproteus sp., Plasmodium sp. and Leucocytozoon sp.). In all, 2 μL of the initial PCR product were used for the second PCR, using primers HaemFL and HaemR2L which amplify 479 bp (excluding primers) of Leucocytozoon spp.
The PCR mix was prepared in a total volume of 20 μL containing 10 μL of PCR master mix (OnePCRTM Ultra, Bio-Helix, New Taipei City, Taiwan), 1 μL of each primer (concentration = 10 μM), 6 μL of distilled water and 2 μL of DNA template (concentration < 25 ng/μL). Leucocytozoon sp. strain KU483 [27] and distilled water were used in every run as positive and negative controls, respectively. Thermal profile of nested-PCR was followed as described in the original protocol [20]. The amplicons were visualised by electrophoresis in 1.5% agarose gel containing the nucleic acid staining (RedSafeTM, iNtRON Biotechnology, Korea). Finally, the positive bands were cut and submitted to the U2Bio Thailand (Bangkok, Thailand) for gel extraction, DNA purification and Sanger sequencing.
Sequence and phylogenetic analysis
Sequences of Leucocytozoon sp. were analysed using BioEdit, version 7.0.5.3 [18]. Electropherograms were checked for the presence of double peaks, indicating co-infection [5], which were excluded for subsequent sequence and phylogenetic analysis. Of note, identification of lineages from multiple strains was not available in this study. Only samples showing single infections had their forward and reverse strands aligned to obtain consensus sequences. The consensus sequences (479 bp) were compared with other known lineages deposited in the MalAvi database [4] using BLAST. A sequence with at least one nucleotide difference was identified as a new lineage [5, 9]. The new lineage was then named and deposited in the MalAvi database. If the analysed sequences showed 100% match with known lineages, they were named as their identical lineage. All the sequences from this study were deposited in GenBank (accession numbers PQ560893, PQ560895, PQ560899, PQ560906-08, PQ560910, PQ560911, PQ560916, and PQ880117).
For Bayesian phylogenetic analysis, 32 cytb sequences of Leucocytozoon sp. deposited in MalAvi database [4] and four sequences from this study were used. The phylogenetic tree was rooted with sequences of Haemoproteus columbae and Haemoproteus iwa. Bayesian phylogenetics was calculated with MrBayes, version 3.2.6 [44], and the analysis was run for three million generations and every 100 trees were sampled. The first 25% of trees were discarded as burn-in and the remaining trees (37,500) were used to generate the consensus tree. According to the hierarchical likelihood ratio tests (hLRTs), the best substitution model for all alignments was general time-reversible (GTR). Genetic divergence was calculated using the Jukes-Cantor model, implemented in MEGA11 [54].
Statistical analysis
The prevalence of Leucocytozoon sp. was calculated based on the results of both microscopic and molecular analysis. The confidence intervals (95% CI) were calculated using the function “binom.approx” in the “epitools” package. Logistic regression was applied to determine the associations between prevalence of Leucocytozoon sp.-infected chickens with sex (male and female) and localities (Kalasin, Roi-Et and Sakon Nakhon). Significance was obtained at a p < 0.05. Student’s t-test was used to evaluate the morphometric differences of fusiform gametocytes between the GALLUS17 and GALLUS63 lineages, with statistical significance determined at p < 0.05. All statistical analyses were implemented in R, version 4.3.0 [43].
Results
Prevalence of Leucocytozoon infection
Blood smear examination revealed 85% (95% CI: 75.97%–94.03%) prevalence of Leucocytozoon infection in chickens (Table 1). The nested-PCR showed slightly higher prevalence of 90% (95% CI: 82.41%–97.59%). Generally, most of the infected chickens had a moderate level of parasitaemia, ranging from 0.01% to 0.16% in 82.35% of the infected chickens. Eight infected chickens had low parasitaemia (<0.01%) and one infected chicken had high parasitaemia (1.77%). Based on microscopic examination, Leucocytozoon sp. (gametocytes in fusiform host cells) and L. macleani were present in 40 and 9 individuals, respectively.
Microscopic examination of parasite infection in chickens in Thailand.
After microscopic examination of 51 fresh blood smear, one fighting cock and four native chickens had a single infection by L. macleani, whereas one fighting cock and three native chickens had double infection of L. macleani and microfilaria. Of note, infections by L. macleani were confirmed by combining microscopic and molecular analysis. Twelve native chickens had a single infection of Leucocytozoon sp. Seven fighting cocks and 20 native chickens had co-infection of Leucocytozoon sp. with other blood parasites, including one with Leucocytozoon-Plasmodium, six with Leucocytozoon-Trypanosoma, and 20 with Leucocytozoon-microfilaria. Furthermore, three native chickens had triple co-infection, one with Leucocytozoon-Plasmodium-Trypanosoma and two with Leucocytozoon-Trypanosoma-microfilaria.
The logistic regression (Table 2) indicated that male chickens had a 2.07 times higher risk of Leucocytozoon infection than female chickens (p = 0.391). The chickens raised in Roi-Et and Sakon Nakhon had a lower risk of Leucocytozoon infection than those raised in Kalasin (OR = 4.98×10⁻⁹ and 2.13×10⁻⁸, respectively; both p = 0.993).
Logistic regression analysis of associations between prevalence of Leucocytozoon spp. and chicken sexes and localities.
Morphological characteristics
Based on the morphological and molecular characteristics, only nine samples were identified as L. macleani single strain infection (Table 3). Of these nine L. macleani samples, only those with parasitaemia above 0.01% were included for further morphometric measurements. Gametocytes (both macrogametocytes and microgametocytes) developed in roundish (Figs. 2E, 2F) and fusiform (Figs. 2A–2D, 2G, 2H) host cells. Macrogametocytes contained a few small vacuoles (Fig. 2A). In fusiform cells, the nuclei of the host cells were usually pushed aside, and lay peripheral as cap-like structures (Fig. 2A, 2C, 2G, 2H). The length of the nuclei of the host cells in GALLUS17, and GALLUS63 was less than 1/3 of the circumferences of gametocytes (Table 4). Nucleus of the parasite (Fig. 2B) was variable both in form and position, with a prominent nucleolus (Fig. 2A). The cytoplasm was stained deep blue, which formed two processes at the ends of gametocytes (Fig. 2A). Length of the cytoplasmic processes was bigger than width.
 |
Figure 2 Gametocytes of Leucocytozoon macleani, lineages GALLUS17 (A–F) and lineages GALLUS63 (G–H). Leucocytozoon macleani gametocytes develop in two types of host cells, including fusiform (A–D, G–H) and roundish (E–F). Cytoplasm of macrogametocytes (A–B, E, G) usually contains small vacuoles (back arrowhead). Nucleus of macrogametocyte (arrow) contains nucleolus (white arrowhead). Two cytoplasmic processes (CP) present in fusiform host cells. Cytoplasm (C) of roundish host cells is largely occupied by macrogametocytes. Both fusiform and roundish host cells with microgametocytes have features similar to host cells with macrogametocytes. Nuclei of microgametocytes are diffuse and cytoplasm stains paler than in macrogametocytes. In fusiform host cell, macrogametocytes and microgametocytes usually displace the nucleus of host cell (double arrow) toward the periphery (A, C, G–H). In fusiform host cells, macrogametocytes and microgametocytes push nuclei aside, deforming them; the nuclei assume band-like form and lie peripherally like a band extending more than half of the circumference of gametocytes. Methanol-fixed, Giemsa-stained blood films. Scale bar = 10 μm. |
Species identification of Leucocytozoon found in Thai chickens (Gallus gallus domesticus).
Morphometry of gametocytes and host cells in two lineages of Leucocytozoon macleani from chickens in Thailand.
In roundish cells, nuclei of the host cells were deformed, pushed peripherally and lied usually as a band. Host cell nuclei covered more than half of the circumference of gametocytes. Nucleus had a prominent nucleolus (Fig. 2E). The cytoplasm of roundish host cell was largely replaced by gametocytes, which usually was found around gametocyte as pale margin of variable form (Fig. 2E).
Microgametocytes of L. macleani showed diffused nucleus that was variable in location. The cytoplasm was stained pale purple (Figs. 2C, 2D, 2F, 2H). In fusiform cells, two cytoplasmic processes were seen, and the cytoplasm rarely contained vacuoles. Length of the cytoplasmic processes was bigger than width. Host cell nucleus was usually pushed aside; it lied periphery (Fig. 2C). Nucleus of fusiform host cell had the length less than 1/3 of the circumferences of gametocytes (Table 4).
Roundish microgametocytes were rarely found. Gametocyte displaced the nucleus of host cell toward the periphery (Fig. 2F). Nucleus of host cell had the band-shaped appearance with the length greater than half of the circumference of gametocyte. The cytoplasm of roundish host cell was largely replaced by gametocytes, which was similar to that of macrogametocytes.
Morphometric analysis showed that some parameters of fusiform and roundish macrogametocytes of L. macleani GALLUS17 (Table 4) fell within the min – max range of L. macleani from the previous reports (Table 5). However, the size of the nucleus of roundish host cells in L. macleani GALLUS17 (mean ± SD = 24.57 ± 3.45 μm; and min – max = 20.03 – 32.14 μm) was greater than reported in previous studies (8.20 – 22.20 μm). In regard to L. macleani GALLUS63, morphometrics of fusiform gametocytes fell within the min – max range of L. macleani from the previous reports.
Morphometry of gametocytes and host cells of Leucocytozoon macleani from previous reports.
Additionally, length of cytoplasmic processes of macrogametocytes and microgametocytes in L. macleani GALLUS63 were significantly shorter than those of L. macleani GALLUS17 (Table 4). The width of cytoplasmic processes in microgametocytes of GALLUS63 was significantly broader than that of GALLUS17, p < 0.05. The area of cytoplasmic processes in macrogametocytes of GALLUS63 was significantly smaller than that of GALLUS17, p < 0.05. The length of the host-parasite complex of macrogametocytes and microgametocytes in GALLUS63 was significantly smaller than that of GALLUS17, p < 0.05. The area of the host-parasites complex of macrogametocytes in GALLUS63 was significantly smaller than that of GALLUS17, p < 0.05.
Molecular characteristics and phylogenetics
The sequence analysis from 54 samples revealed that most chickens were co-infected with multiple Leucocytozoon lineages (n = 44). Only ten chickens were infected by a single Leucocytozoon lineage, which were identified as four lineages as GALLUS17, GALLLUS34, GALLUS63, and ASIFLA01 (Table 3). The lineage GALLUS63 was the novel lineage and was isolated from a chicken (samples: ROE16). Additionally, the most common lineage found in this study was the GALLUS17, which was found in seven chickens (both fighting cock and native chickens) raised in Kalasin (samples: KLS03 and KLS05), Roi-Et (samples: ROE12 and ROE17) and Sakhon Nakhon (samples: SKN07, SKN08 and SKN14).
The Bayesian phylogenetic inference (Fig. 3) of cytb gene revealed that L. macleani lineages GALLUS17, GALLUS34, and GALLUS63 grouped separately from L. macleani GALLUS08 isolated from chicken in Malaysia and other two L. macleani isolated from chickens in Thailand and Malaysia. However, our three L. macleani sequences GALLUS17, GALLUS34, and GALLUS63 grouped with other Leucocytozoon spp. lineages obtained from chickens in Thailand, GALLUS52, GALLUS60, and GALLUS61. The genetic homology in this clade was 97.30%–99.57%. Furthermore, our L. macleani (clade B) had 79.85%–82.07% genetic similarity with other L. macleani (clade A), 85.20%–86.84% genetic similarity with Leucocytozoon caulleryi and 90.35%–92.77% genetic similarity with Leucocytozoon schoutedeni. Additionally, one Leucocytozoon sequence isolated in this study was identified as lineage ASIFLA01. This lineage was grouped together with Leucocytozoon danilewskyi lineage BUBP01, which was previously reported from the Eurasian eagle-owl (Bubo bubo) in Spain (Fig. 3). Our Leucocytozoon sp. ASIFLA01 showed 98.70% similarity to L. danilewskyi BUBP01.
 |
Figure 3 Bayesian phylogeny constructed by using the cytochrome b sequences (479 bp) of Leucocytozoon parasites, including four sequences isolated from village chickens in Thailand (orange bold text) and other sequences deposited in the MalAvi database. Other Leucocytozoon lineages isolated from chickens are given in purple. Clade A contains sequences of Leucocytozoon macleani reported in other studies, while clade B contains sequences of L. macleani isolated from this study. MalAvi lineage codes and GenBank accession number are given after species name. Node values (in percentage) indicate the posterior clade probabilities. |
Discussion
Blood parasites in poultry are often neglected; however, their prevalence can be especially high in hot and humid regions, such as Thailand. According to the reported exo-erythrocytic stages of L. macleani, merogony takes places in hepatocytes, brain capillary endothelial cells, and renal tubular cells [58, 62]. Generally, the pathogenicity of most Leucocytozoon species appears to be associated with the presence or absence of megalomeronts, which are often encapsulated by fibrotic tissue and surrounded by mixed inflammatory infiltrates, including erythrocytes, plasma cells, macrophages, and heterophils. Finally, these megalomeronts might undergo necrosis and calcification [2].
Atkinson et al. (1991) also explained that Leucocytozoon species can cause severe anaemia through the destruction of infected blood cells by the reticuloendothelial cells in the spleen, as well as by the destruction of uninfected erythrocytes by anti-erythrocyte factors appearing in the serum during acute infection. However, the megalomeronts were not found in Leucocytozoon macleani [62]. Additionally, due to high prevalence of Leucocytozoon parasites and the frequent occurrence of multiple-strain infections in this study, further targeted investigation is required before any conclusions on pathology of L. macleani infections in Thai chickens. As our first step in research of chicken leucocytozoonosis, this study only explained detailed molecular and morphological characteristics of L. macleani that are prevalent in this area. The finding showed that prevalence of leucocytozoonosis detected by PCR (90%) was slightly higher than that of microscopy (85%). The authors suggested using microscopic method in routine laboratory diagnosis of chicken leucocytozoonosis, as it is an inexpensive method suitable for use in underdeveloped and developing countries.
The prevalence of leucocytozoonosis was high in Thai domestic chickens (Gallus gallus domesticus) similarly to the previous reports in the country [5, 25]. That highlighted the need for continuous monitoring and challenges for the elimination of leucocytozoonosis. Additionally, it underscored the importance of further investigation to identify potential transmission of leucocytozoonosis agents to the larger commercial poultry production sector. Furthermore, it might be worth conducting further studies to precisely measure the economic impact of leucocytozoonosis as well, particularly due to damage to internal organs by tissue stages, which remain insufficiently investigated both in L. schoutedeni and L. macleani, especially in juvenile birds.
The majority of infected chickens (42 out of 51 infected chickens) had moderate level of parasitaemia (>0.01% to 1%). The level of parasitaemia might be associated with host population density, as a higher density could increase the transmission rate and promote infection by different strains, ultimately leading to increased parasitaemia [33]. Thus, implementing flock size manipulation might reveal evidence that supports this assumption. On the other hand, the intensity of parasitaemia might depend on circadian rhythms. Chagas et al. [8] described the circadian patterns in avian blood parasites, including Plasmodium, Leucocytozoon species, Trypanosoma species and microfilariae, with Leucocytozoon species peaking mainly during the evening and night. Additionally, the level of parasitaemia might be influenced by exposure to potential vectors. In a previous study in Boreal Owls (Aegolius funereus) infected by Leucocytozoon parasites, it was shown that males, which hunt prey for their mate and nestlings during incubation, had higher levels of parasitaemia than females, which typical remain in cavity nests [49]. Although chickens with moderate parasitaemia were likely invulnerable to Leucocytozoon infection, these infections cannot be neglected, mainly because in favourable conditions, these infections might lead to severe illness. We assumed that parasitaemia intensity might be related to the tissue merogony stage and the patterns of persistence in avian hosts. In another haemosporidian parasite, Haemoproteus, the parasitaemia level did not necessarily correlate with the abundance of tissue merogony, and the presence of parasitaemia was not an indicator of the existence of tissue merogony [12]. However, this phenomenon remains insufficiently studied in chickens, highlighting the need for further research.
Multiple blood parasites and multiple Leucocytozoon lineage infections were frequently observed in this study, with microscopic examination revealing the presence of up to three different blood parasites in a single host. Blood parasites are transmitted by various blood-sucking insects. For example, L. macleani and L. schoutedeni are transmitted by blackflies, while L. caulleryi is transmitted by biting midges [58]. Plasmodium gallinaceum and P. juxtanucleare are known to be transmitted by Culex mosquitoes [5, 42], whereas Trypanosoma spp. are carried by mosquitoes, hippoboscid flies, black flies, biting midges, and mites [34, 38, 51, 55, 69]. Microfilariae were transmitted by blackflies, biting midges, mosquitoes, and tabanids [1, 16, 40, 47]. Isolation of multiple parasites from a single bird in this study indicated the presence of diverse vectors and transmission of different vector-borne pathogens in the area; however, this was not investigated in the current study.
Based on analysis of electropherogram, the presence of double peaks in cytb sequences indicated infections by multiple Leucocytozoon lineages. This approach for identification of mixed-lineages infection has been used in several studies, including a study of Leucocytozoon sp. and Plasmodium sp. in Gallus gallus domesticus [5]; a study of Leucocytozoon sp. in Passeriformes birds [15]; a study of Leucocytozoon sp., Plasmodium sp., and Haemoproteus sp. in Accipitriformes birds [19]; a study of haemosporidian parasites in Accipitriformes and Strigiformes birds [37, 39]; a study of haemosporidian parasites in Passeriformes birds [6]; and a study of haemosporidian parasites in Passeriformes, Coraciiformes, Columbiformes, and Piciformes birds [24]. The presence of these mixed infections make lineage description based on the MalAvi nomenclature [4] impossible. In this study, only single lineage infections were assigned the lineage.
Sequencing of cytb gene fragments revealed that most chickens were infected with multiple Leucocytozoon lineages, except for ten chickens. This finding highlighted the importance of careful identification and description of Leucocytozoon in chickens. For this reason, it was suggested to combine microscopy and molecular techniques for identification of Leucocytozoon parasites for species description. Although various compounds (e.g., clopidol, primaquine, halofuginone polystyrene sulfonate, and sulfamonomethoxine and ormetoprim combinations) have been used to treat Leucocytozoon infections, their efficacy remains debated [68]. Furthermore, Zhao et al. (2016) found that sulfaquinoxaline or pyrimethamine had no or limited effects on L. macleani infections. Sulfamonomethoxine at concentration of 30 and 40 ppm can be used for the treatment of L. caulleryi leucocytozoonosis in poultry [29]. Sulfamonomethoxine is a sulfonamide antibiotic inhibiting dihydropteoate synthetase (DHPS), which is an important enzyme for the folate pathway of bacteria and primitive eukaryotes [67]. The products from this pathway are essential for DNA synthesis [22], which might influence asexual multiplication at the merogony stage of leucocytozoids.
Nine out of ten chickens with single infection were infected by Leucocytozoon parasites that developed both in fusiform and roundish host cells, which were the characteristics of L. macleani. In contrast, L. caulleryi and L. schoutedeni developed exclusively in roundish host cells [58]. Leucocytozoon macleani fusiform macrogametocytes exhibited characteristic features resembling those previously described, such as cytoplasm containing small vacuoles, a host cell nucleus extending less than 1/3 of the circumference of gametocyte, and the cytoplasmic processes longer than their width [45, 58]. However, in roundish cells, the nucleus extended beyond half of the gametocyte’s circumference. This differed from the previous description, in which nucleus of roundish host cell extended less than half of the gametocyte’s circumference [58]. From the morphometric point of view, it was found that the length of nucleus of roundish host cells in this study (Table 3) was longer than the previous report (Table 4). Additionally, there was a significant difference in the length of cytoplasmic processes and length of host-parasite complex in L. macleani lineages. These distinct characteristics might provide evidence for a species complex within L. macleani, a phenomenon previously described in Leucocytozoon toddi, which developed within fusiform host cells of diurnal raptors [61].
More recently, Valkiūnas et al. (2010) identified two populations of L. toddi with different lengths of cytoplasmic processes, supported by the genetic divergence of the cytb gene between these two groups. Together with the different avian host, L. toddi in these two populations was split into Leucocytozoon mathisi and Leucocytozoon buteonis. In this study, the difference in morphologic characteristics (Fig. 2) was supported by phylogenetic analysis (Fig. 3) and genetic divergence among cytb sequences of our isolated L. macleani lineages, 1.3% to 2.19% genetic distances. Thus, L. macleani GALLUS17 and GALLUS63 (new lineage identified in this study) might represent a case of cryptic speciation (these two lineages showed 1.74% genetic distance). However, it was not within the scope of this study to describe a new subspecies, which requires further investigation, including type of host cell infected by the parasite, parasite biology, and detailed molecular information [15].
This study revealed the common L. macleani lineages in Thailand, which was lineage GALLUS17. Previously, this lineage was also found in Southern Thailand [5]. Thus, L. macleani GALLUS17 might be the important leucocytozoonosis agent causing the negative impact in chickens in a wider geographical area. Together with our finding of the existence of a new lineage, L. macleani GALLUS63 and previous reports of several lineages of both described and non-described species in Thailand [52], it can be assumed that there is high genetic diversity of Leucocytozoon sp. in the country. This study assumed uncertainty regarding the pathology of L. macleani infection in Thai chicken due to the frequent occurrence of multiple Leucocytozoon-strain infections. On a different note, L. macleani had been reported to be of low virulence [48, 58], which might enable the parasite to produce multiple successive generations and gradually drive genetic change over the time. The relationship between lineage diversity and evolution in haemosporidian parasites was previously discussed by Huang et al. [21]. Thus, the authors suggested that L. macleani is a suitable model organism for such research, due to its high lineage diversity and the ease of conducting experimental studies with its vertebrate host.
Additionally, this study was able to retrieve one sequence of owl Leucocytozoon sp. from chicken blood, which was the Leucocytozoon sp. lineage ASIFLA01 (GenBank accession No: PQ880117). This lineage ASIFLA01 was originally described from Leucocytozoon sp. isolated from Short-eared Owl (Asio flammeus) in Japan (GenBank accession No: LC230137) [23]. A previous study also reported the detection of wild bird haemosporidian DNA in chicken blood, and our study supported that finding. Chatan et al. [10] reported the Plasmodium sp. lineage ACCBAD01, FANTAIL01, and ORW1, which were originally described from Shikra (Accipiter badius, GenBank No: JN639001), Australian Rufous Fantail (Rhipidura rufifrons, GenBank No: AY714196), and Oriental Reed Warbler (Acrocephalus orientalis, GenBank No: AF254963), respectively. This phenomenon was considered an abortive infection, where the parasite invaded the wrong host, but failed to complete its entire life cycle [62].
Last but not least, Bayesian phylogenetics revealed two clades of L. macleani (Fig. 3). Clade A, containing L. macleani isolated from chickens raised in Malaysia and Thailand, showed 17.93% to 20.15% genetic distance from clade B containing our L. macleani. Furthermore, clade A was grouped closely with Leucocytozoon lovati lineage LAMUT01 reported by Sato et al. [46], with 18.48% to 19.03% genetic distances, whereas clade B had 16.84% to 17.93% genetic distance from L. lovati. Morphologically, L. macleani and L. lovati were very similar [58]. The length of the host cell-parasite complex in L. lovati ranged from 22.0 to 60.8 μm (mean ± SD: 42.7 ± 8.94 μm) for macrogametocytes and 5.6 to 60.8 μm (mean ± SD: 45.9 ± 9.17 μm) for microgametocytes [17]. In this case, the authors found high genetic distance between L. macleani and L. lovati. However, based on Bayesian phylogenetic analysis, L. lovati might be able to infect chickens and could be a novel pathogen of poultry.
Conclusion
This study revealed a high prevalence of Leucocytozoon infections in chickens in Thailand, calling for more research on these pathogens in poultry. It is the first report describing lineages in Leucocytozoon macleani (synonym Leucocytozoon sabrazesi) in Southeast Asia, following the MalAvi database nomenclature, together with the detailed morphological characteristics of gametocytes and their host cells. A new lineage of L. macleani was identified (GALLUS63). Furthermore, our findings suggested potential cryptic speciation within Leucocytozoon macleani. This study identified many multiple Leucocytozoon-strain infections, highlighting the importance of careful identification and description of these parasites in chickens. Altogether, this information can serve as a valuable reference for routine veterinary laboratory diagnostics and future investigation. Research on the exo-erythrocytic development of chicken leucocytozoids remains in its infancy but is essential for better understanding pathology during poultry leucocytozoonosis.
Acknowledgments
The authors wish to thank Dr. Gediminas Valkiūnas for his valuable suggestion during the preparation of this manuscript. Additionally, we thank the livestock authorities and local farmers for their cooperation in sample collection processes.
Funding
This work was supported by Walailak University under the New Researcher Development scheme (Contract Number WU67211).
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Anderson RC. 2000. Nematode parasites of vertebrates: their development and transmission, 2nd edn. Wallingford: CAB International. [Google Scholar]
- Atkinson CT, van Riper III C. 1991. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus, in Bird-Parasite Interactions: Ecology, Evolution, and Behaviour, Loye JE, Zuk M, Editors, Oxford University Press: New York. p. 19–48. [Google Scholar]
- Beckstead R. 2020. Miscellaneous and sporadic protozoal infections, in Diseases of Poultry, 14th edn, Swayne DE, Editor, John Wiley & Sons: New Jersey. p. 1231–1254. [Google Scholar]
- Bensch S, Hellgren O, Pérez-Tris J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources, 9, 1353–1358. [CrossRef] [PubMed] [Google Scholar]
- Boonchuay K, Thomrongsuwannakij T, Chagas CRF, Pornpanom P. 2023. Prevalence and diversity of blood parasites (Plasmodium, Leucocytozoon and Trypanosoma) in backyard chickens (Gallus gallus domesticus) raised in Southern Thailand. Animals, 13, 2798. [Google Scholar]
- Cadena-Ortiz H, Mantilla JS, de Aguilar JR, Flores D, Bahamonde D, Matta NE, Bonaccorso E. 2019. Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: diversity and factors related to prevalence and parasitaemia. Parasitology, 146, 765–773. [Google Scholar]
- Campbell JG. 1954. Bangkok haemorrhagic disease of chickens: An unusual condition associated with an organism of uncertain taxonomy. Journal of Pathology and Bacteriology, 68, 423–429. [Google Scholar]
- Chagas CRF, Binkienė R, Valkiūnas G. 2021. Description and molecular characterization of two species of avian blood parasites, with remarks on circadian rhythms of avian haematozoa infections. Animals, 11, 3490. [Google Scholar]
- Chagas CRF, Duc M, Gutiérrez-Liberato GA, Valkiūnas G. 2023. Host cells of Leucocytozoon (Haemosporida, Leucocytozoidae) gametocytes, with remarks on the phylogenetic importance of this character. Pathogens, 12, 712. [Google Scholar]
- Chatan W, Khemthong K, Akkharaphichet K, Suwarach P, Seerintra T, Piratae S. 2024. Molecular survey and genetic diversity of Plasmodium sp. infesting domestic poultry in Northeastern Thailand. Journal of Veterinary Research, 68, 101–108. [Google Scholar]
- Chiang Y-H, Lin Y-C, Wang S-Y, Lee YP, Chen C-F. 2022. Effects of Artemisia annua on experimentally induced leucocytozoonosis in chickens. Poultry Science, 101, 101690. [Google Scholar]
- Duc M, Himmel T, Ilgūnas M, Eigirdas V, Weissenböck H, Valkiūnas G. 2023. Exo-erythrocytic development of two Haemoproteus species (Haemosporida, Haemoproteidae), with description of Haemoproteus dumbbellus, a new blood parasite of bunting birds (Emberizidae). International Journal for Parasitology, 53, 531–543. [CrossRef] [PubMed] [Google Scholar]
- Gimba F, Zakaria A, Mugok LB, Siong HC, Jaafar N, Mokhtar MA, Rahman ARA, Amzah A, Abu J, Sani RA, Babjee SM, Sharma RS. 2014. Haemoparasites of domestic poultry and wild birds in Selangor, Malaysia. Malaysian Journal of Veterinary Research, 5, 43–51. [Google Scholar]
- Groff TC, Lorenz TJ, Iezhova TA, Valkiūnas G, Sehgal RNM. 2022. Description and molecular characterization of novel Leucocytozoon parasite (Apicomplexa: Haemosporida: Leucocytozoidae), Leucocytozoon polynuclearis n.sp. found in North American woodpeckers. Systematic Parasitology, 99, 103–114. [Google Scholar]
- Gutiérrez-Liberato GA, Duc M, Eigirdas V, Chagas CRF. 2025. Leucocytozoon infections in tits (Aves, Paridae): blood and tissue stages investigated using an integrative approach. Parasite, 32, 13. [Google Scholar]
- Haas M, Baruš V, Benedikt V, Literák I. 2011. Microfilariae in birds in the Czech Republic, including a note on adult nematodes Eufilaria delicata in a song thrush Turdus philomelos. Parasitology Research, 109, 645–655. [Google Scholar]
- Hagihara M, Yamaguchi T, Kitahara M, Hirai K, Murata K. 2004.Leucocytozoon lovati infections in Wild Rock Ptarmigan (Lagopus mutus) in Japan. Journal of Wildlife Diseases, 40, 804–807. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Harl J, Himmel T, Valkiūnas G, Ilgūnas M, Nedorost N, Matt J, Kübber-Heiss A, Alic A, Konicek C, Weissenböck H. 2022. Avian haemosporidian parasites of accipitriform raptors. Malaria Journal, 21, 14. [CrossRef] [PubMed] [Google Scholar]
- Hellgren O, Waldenström J, Bensch S. 2004. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avain blood. Journal of Pasasitology, 90, 797–802. [Google Scholar]
- Huang X, Dong L, Zhang C, Zhang Y. 2015. Genetic diversity, temporal dynamics, and host specificity in blood parasites of passerines in north China. Parasitology Research, 114, 4513–4520. [Google Scholar]
- Hyde JE. 2005. Exploring the folate pathway in Plasmodium falciparum. Acta Tropica, 94, 191–206. [Google Scholar]
- Inumaru M, Murata K, Sato Y. 2017. Prevalence of avian haemosporidia among injured wild birds in Tokyo and environs, Japan. International Journal for Parasitology: Parasites and Wildlife, 6, 299–309. [Google Scholar]
- Ivanova K, Zehtindjiev P, Mariaux J, Georgiev BB. 2015. Genetic diversity of avian haemosporidians in Malaysia: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Selangor. Infection, Genetics and Evolution, 31, 33–39. [Google Scholar]
- Khumpim P, Chawengkirttikul R, Junsiri W, Watthanadirek A, Poolsawat N, Minsakorn S, Srionrod N, Anuracpreeda P. 2021. Molecular detection and genetic diversity of Leucocytozoon sabrazesi in chickens in Thailand. Scientific Reports, 11, 16686. [Google Scholar]
- Kitaoka S. 1978. Serological diagnosis of chicken leucocytozoonosis. Japan Agricultural Research Quarterly, 12, 157–160. [Google Scholar]
- Lertwatcharasarakul P, Salakij C, Prasopsom P, Kasorndorkbua C, Jakthong P, Santavakul M, Suwanasaeng P, Ploypan R. 2021. Molecular and morphological analyses of Leucocytozoon parasites (Haemosporida: Leucocytozoidae) in raptors from Thailand. Acta Parasitologica, 66, 1406–1416. [Google Scholar]
- Morii T, Shiihara T, Lee YC, Manuel MF, Nakamura K, Iijima T, Hoji K. 1981. Seroimmunological and parasitological surveys of Leucocytozoon caulleryi infection in chickens in several Asian countries. International Journal for Parasitology, 11, 187–190. [CrossRef] [PubMed] [Google Scholar]
- Nakamura K, Morii T, IIjima T. 1979. Effects of sulfamonomethoxine on parasitemia, serum antigen and antibody production in chickens infected with Leucocytozoon caulleryi. Japanese Journal of Parasitology, 28, 377–383. [Google Scholar]
- Nakamura K, Mitarai Y, Tanimura N, Hara H, Ikeda A, Shimada J, Isobe T. 1997. Pathogenesis of reduced egg production and soft-shelled eggs in laying hens associated with Leucocytozoon caulleryi infection. Journal of Parasitology, 83(2), 325. [Google Scholar]
- Nakamura K. 2022. Leucocytozoon caulleryi infection in chickens: Etiology, pathology, and diagnosis. Japan Agricultural Research Quarterly, 56, 121–127. [Google Scholar]
- Nawata E, Nagata Y, Sasaki A, Iwama K, Sakuratani T. 2005. Mapping of climatic data in Northeast Thailand: Rainfall. Tropics, 14, 191–201. [Google Scholar]
- Ortego J, Cordero PJ. 2010. Factors associated with the geographic distribution of leucocytozoa parasitizing nestling eagle owls (Bubo bubo): A local spatial-scale analysis. Conservation Genetics, 11, 1479–1487. [Google Scholar]
- Peirce MA. 2016. Infectious diseases, in Avian Medicine, 3rd edn, Samour J, Editor, Elservier: Missouri, p. 434–521. [Google Scholar]
- Piratae S, Vaisusuk K, Chatan W. 2021. Prevalence and molecular identification of Leucocytozoon spp. in fighting cocks (Gallus gallus) in Thailand. Parasitology Research, 120, 2149–2155. [CrossRef] [PubMed] [Google Scholar]
- Pohuang T, Jittimanee S, Junnu S. 2021. Pathology and molecular characterization of Leucocytozoon caulleryi from backyard chickens in Khon Kaen Province, Thailand. Veterinary World, 14, 2634–2639. [Google Scholar]
- Pornpanom P, Chagas CRF, Lertwatcharasarakul P, Kasorndorkbua C, Valkiūnas G, Salakij C. 2019. Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: Data from a rehabilitation centre. International Journal for Parasitology: Parasites and Wildlife, 9, 248–257. [Google Scholar]
- Pornpanom P, Salakij C, Prasopsom P, Lertwatcharasarakul P, Kasorndorkbua C. 2019. Morphological and molecular characterization of avian trypanosomes in raptors from Thailand. Parasitology Research, 118, 2419–2429. [Google Scholar]
- Pornpanom P, Kasorndorkbua C, Lertwatcharasarakul P, Salakij C. 2021. Prevalence and genetic diversity of Haemoproteus and Plasmodium in raptors from Thailand: Data from rehabilitation center. International Journal for Parasitology: Parasites and Wildlife, 16, 75–82. [Google Scholar]
- Pornpanom P, Boonchuay K. 2024. Preliminary study on buffy coat smear and molecular detection of microfilaria in domestic chickens (Gallus gallus domesticus) raised in Southern Thailand. Veterinary World, 17, 888–894. [Google Scholar]
- Pornpanom P, Valkiūnas G, Paudel S. 2025. Morphological and molecular characterization of avian trypanosomes in domestic chickens (Gallus gallus domesticus) in Southeast Asia and review of the parasite morphometry in different avian hosts. Avian Pathology, 54, 489–497. [Google Scholar]
- Pruck-Ngern M, Pattaradilokrat S, Chumpolbanchorn K, Pimnon S, Harnyuttanakorn P, Buddhirakkul P, Saiwichai T. 2014. Refractoriness of the natural malaria vector Culex quinquefasciatus to Plasmodium gallinaceum. Journal of Tropical Medicine and Parasitology, 37, 60–68. [Google Scholar]
- R Core Team. 2024. R: A language and environment for statistical computing. Available from: https://www.R-project.org/. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. [CrossRef] [PubMed] [Google Scholar]
- Sacchi L, Prigioni C. 1985. Haematozoa of Italian birds. 1: Redescription of Leucocytozoon macleani Sambon, 1908 (Apicomplexa Haemosporina) from Phasianus colchicus. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, 126, 89–93. [Google Scholar]
- Sato Y, Hagihara M, Yamaguchi T, Yukawa M, Murata K. 2007. Phylogenetic comparison of Leucocytozoon spp. from wild birds of Japan. Journal of Veterinary Medical Science, 69, 55–59. [CrossRef] [PubMed] [Google Scholar]
- Sehgal RNM, Jones HI, Smith TB. 2005. Molecular evidence for host specificity of parasitic nematode microfilariae in some African rainforest birds. Molecular Ecology, 14, 3977–3988. [Google Scholar]
- Sehgal RNM, Valkiunas G, Iezhova TA, Smith TB. 2006. Blood parasites of chickens in Uganda and Cameroon with molecular descriptions of Leucocytozoon schoutedeni and Trypanosoma gallinarum. Journal of Parasitology, 92, 1336–1343. [Google Scholar]
- Ševčík R, Mahlerová K, Riera FA, Zárybnická M. 2024. Leucocytozoon infection does not influence the survival of Boreal Owl Aegolius funereus nestlings. Avian Diseases, 68, 134–140. [Google Scholar]
- Suprihati E, Kusnoto K, Triakoso N, Yuniarti WM. 2020. Histopathological studies on Leucocytozoon caulleryi Infection on broiler in endemic area of Indonesia. Systematic Reviews in Pharmacy, 11, 1219–1223. [Google Scholar]
- Svobodová M, Dolnik OV, Čepička I, Rádrová J. 2017. Biting midges (Ceratopogonidae) as vectors of avian trypanosomes. Parasites & Vectors, 10, 224. [Google Scholar]
- Swangneat K, Srikacha N, Soulinthone N, Paudel S, Srisanyong W, James Stott CJ, Mahawan T, Pornpanom P. 2025. Molecular prevalence of avian haemosporidian parasites in Southeast Asia: Systematic review and meta-analysis. Animals, 15, 636. [Google Scholar]
- Takang P, Pikulkaew S, Awaiwanont N, Numee S. 2017. Prevalence and risk factors of blood parasites infection in backyard chickens in Chiang Mai. Chiang Mai Veterinary Journal, 15, 157–167. [Google Scholar]
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. [CrossRef] [PubMed] [Google Scholar]
- Thaijarern J, Tangkawanit U, Wongpakam K, Pramual P. 2019. Molecular detection of Trypanosoma (Kinetoplastida: Trypanosomatidae) in black flies (Diptera: Simuliidae) from Thailand. Acta Tropica, 200, 105196. [Google Scholar]
- Thilagavathi K, Selvaraj J, Velusamy R, Prasath NB, Prabu PC, Hariharan J. 2022. Outbreak of leucocytozoonosis in commercial white Leghorn layer chickens. Indian Journal of Veterinary Pathology, 46, 289–294. [Google Scholar]
- Valkiūnas G, Iezhova TA, Mironov SV. 2002. Leucocytozoon hamiltoni n.sp. (Haemosporida, Leucocytozoidae) from the Bukharan great tit Parus bokharensis. Journal of Parasitology, 88, 577–581. [Google Scholar]
- Valkiūnas G. 2005. Avian malaria parasites and other haemosporidia. Boca Raton: CRC Press. [Google Scholar]
- Valkiūnas G, Anwar AM, Atkinson CT, Greiner EC, Paperna I, Peirce MA. 2005. What distinguishes malaria parasites from other pigmented haemosporidians? Trends in Parasitology, 21, 357–358. [Google Scholar]
- Valkiūnas G, Iezhova T-A, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S. 2008. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. Journal of Parasitology, 94, 1395–1401. [CrossRef] [PubMed] [Google Scholar]
- Valkiūnas G, Sehgal RNM, Iezhova TA, Hull AC. 2020. Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids, Journal of Parasitology, 96, 170–177. [Google Scholar]
- Valkiūnas G, Iezhova TA. 2017. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malaria Journal, 16, 101. [CrossRef] [PubMed] [Google Scholar]
- Valkiūnas G, Iezhova TA. 2018. Keys to the avian malaria parasites. Malaria Journal, 17, 212. [Google Scholar]
- Valkiūnas G, Iezhova TA. 2023. Insights into the biology of Leucocytozoon species (Haemosporida, Leucocytozoidae): Why is there slow research progress on agents of leucocytozoonosis? Microorganisms 11, 1251. [CrossRef] [PubMed] [Google Scholar]
- Wiegmann A, Springer A, Rinaud T, Ottensmann M, Legler M, Krüger O, Fehr M, Chakarov N, Strube C. 2021. The prevalence of Leucocytozoon spp. in nestlings of three wild raptor species including implications on haematological and blood chemistry values. International Journal for Parasitology: Parasites and Wildlife, 16, 236–243. [Google Scholar]
- Win SY, Chel HM, Hmoon MM, Htun LL, Bawm S, Win MM, Murata S, Nonaka N, Nakao R, Katakura K. 2020. Detection and molecular identification of Leucocytozoon and Plasmodium species from village chickens in different areas of Myanmar. Acta Tropica, 212, 105719. [CrossRef] [PubMed] [Google Scholar]
- Yun M-K, Wu Y, Li Z, Zhao Y, Waddell MB, Ferreira AM, Lee RE, Bashford D, Stephen W, White SW. 2012. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science, 335, 1110–1114. [Google Scholar]
- Zhao W, Pang Q, Xu R, Liu J, Liu S, Li J, Su X. 2016. Monitoring the prevalence of Leucocytozoon sabrazesi in Southern China and testing tricyclic compounds against gametocytes. PLoS One, 11, e0161869. [Google Scholar]
- Zídková L, Cepicka I, Szabová J, Svobodová M. 2012. Biodiversity of avian trypanosomes. Infection, Genetics and Evolution, 12, 102–112. [Google Scholar]
Cite this article as: Srikacha N, Fernandes Chagas CR, Paudel S & Pornpanom P. 2025. Prevalence, morphological and molecular characterization of Leucocytozoon macleani (Apicomplexa: Haemosporida) from chickens in Thailand. Parasite 32, 50. https://doi.org/10.1051/parasite/2025043.
All Tables
Logistic regression analysis of associations between prevalence of Leucocytozoon spp. and chicken sexes and localities.
Species identification of Leucocytozoon found in Thai chickens (Gallus gallus domesticus).
Morphometry of gametocytes and host cells in two lineages of Leucocytozoon macleani from chickens in Thailand.
Morphometry of gametocytes and host cells of Leucocytozoon macleani from previous reports.
All Figures
 |
Figure 1 Study sites and prevalence of Leucocytozoon species in domestic chickens in Northeastern provinces of Thailand. Kalasin (KLS), Roi-Et (ROE), and Sakon Nakhon (SKN). The figure is generated by QGIS version 3.36.2-Maidenhead. |
| In the text | |
 |
Figure 2 Gametocytes of Leucocytozoon macleani, lineages GALLUS17 (A–F) and lineages GALLUS63 (G–H). Leucocytozoon macleani gametocytes develop in two types of host cells, including fusiform (A–D, G–H) and roundish (E–F). Cytoplasm of macrogametocytes (A–B, E, G) usually contains small vacuoles (back arrowhead). Nucleus of macrogametocyte (arrow) contains nucleolus (white arrowhead). Two cytoplasmic processes (CP) present in fusiform host cells. Cytoplasm (C) of roundish host cells is largely occupied by macrogametocytes. Both fusiform and roundish host cells with microgametocytes have features similar to host cells with macrogametocytes. Nuclei of microgametocytes are diffuse and cytoplasm stains paler than in macrogametocytes. In fusiform host cell, macrogametocytes and microgametocytes usually displace the nucleus of host cell (double arrow) toward the periphery (A, C, G–H). In fusiform host cells, macrogametocytes and microgametocytes push nuclei aside, deforming them; the nuclei assume band-like form and lie peripherally like a band extending more than half of the circumference of gametocytes. Methanol-fixed, Giemsa-stained blood films. Scale bar = 10 μm. |
| In the text | |
 |
Figure 3 Bayesian phylogeny constructed by using the cytochrome b sequences (479 bp) of Leucocytozoon parasites, including four sequences isolated from village chickens in Thailand (orange bold text) and other sequences deposited in the MalAvi database. Other Leucocytozoon lineages isolated from chickens are given in purple. Clade A contains sequences of Leucocytozoon macleani reported in other studies, while clade B contains sequences of L. macleani isolated from this study. MalAvi lineage codes and GenBank accession number are given after species name. Node values (in percentage) indicate the posterior clade probabilities. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


