| Issue |
Parasite
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 43 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/parasite/2025038 | |
| Published online | 16 July 2025 | |
urn:lsid:zoobank.org:pub:096627ED-6F89-4A64-B5BF-7F29C9701979
Research Article
Discovery of new species of mesoparasitic pennellid (Copepoda: Siphonostomatoida) from the endemic mesopelagic lightfish Vinciguerria mabahiss in the Red Sea
Découverte d’une nouvelle espèce de Pennellidae mésoparasite (Copepoda : Siphonostomatoida) du poisson bioluminescent mésopélagique endémique Vinciguerria mabahiss en mer Rouge
1
Marine Science Program, Biological and Environmental Science and Engineering Division (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia
2
National Center for Wildlife (NCW), PP2R + WJH, Makkah Al Mukarramah Branch Rd, King Abdul Aziz, Riyadh 12411, Kingdom of Saudi Arabia
3
OceanX, 37 West 39th St, 8th Floor, New York, NY 10018, USA
4
Graduate School of Science and Engineering, Kagoshima University, 1-21-35 Korimoto, Kagoshima City, Kagoshima 890-0065, Japan
* Corresponding author: kahkheng.lim@kaust.edu.sa
Received:
16
February
2025
Accepted:
13
June
2025
A new species of the genus Cardiodectes Wilson, 1917 (Siphonostomatoida: Pennellidae), Cardiodectes tofaili n. sp., is described based on 13 adult females from ten specimens of the endemic lightfish Vinciguerria mabahiss (Stomiiformes: Phosichthyidae). These hosts were inadvertently captured by a remotely operated vehicle at depths of 454–645 m in the pelagic waters of the Saudi Arabian Red Sea. The new species is placed under the “rubosus” group, characterized by possession of a trunk without a discrete abdomen. It is distinguished from its 12 congeners within this group by having a short neck region with a distinct fourth pedigerous somite, and a trunk that is ca. 5 times longer than wide. Phylogenetic analysis based on concatenated 18S + 28S rDNA sequences supports the distinctiveness of the new species. This species is endemic to the Red Sea, representing the first recorded mesoparasite from the mesopelagic environment of the region. This discovery highlights the unique biodiversity of the Red Sea and underscores the importance of exploring mesopelagic ecosystems.
Résumé
Une nouvelle espèce du genre Cardiodectes Wilson, 1917 (Siphonostomatoida : Pennellidae), Cardiodectes tofaili n. sp., est décrite à partir de 13 femelles adultes issues de dix spécimens du poisson bioluminescent endémique Vinciguerria mabahiss (Stomiiformes : Phosichthyidae). Ces hôtes ont été capturés par inadvertance par un véhicule télécommandé à des profondeurs de 454 à 645 m dans les eaux pélagiques de la mer Rouge saoudienne. La nouvelle espèce est classée dans le groupe « rubosus », caractérisé par un tronc sans abdomen distinct. Elle se distingue de ses 12 congénères dans ce groupe par un cou court avec un quatrième somite pédigère distinct, et un tronc environ 5 fois plus long que large. L’analyse phylogénétique basée sur des séquences concaténées d’ADNr 18S + 28S confirme le caractère distinctif de la nouvelle espèce. Cette espèce est endémique de la mer Rouge et constitue le premier mésoparasite recensé dans l’environnement mésopélagique de la région. Cette découverte met en évidence la biodiversité unique de la mer Rouge et souligne l’importance d’explorer les écosystèmes mésopélagiques.
Key words: Phosichthyidae / Mesoparasite / Cardiodectes / Western Indian Ocean
Edited by: Jean-Lou Justine
© K.K. Lim et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The Red Sea harbors a unique marine ecosystem characterized by its warm, deep-sea environment and exceptional biodiversity. Among its inhabitants, mesopelagic fish species, such as the endemic lightfish Vinciguerria mabahiss and the widely distributed skinnycheek lanternfish Benthosema pterotum, stand out as key components of this ecosystem [1, 20]. These species play crucial roles, not only as significant contributors to the vertebrate biomass of the mesopelagic zone [12, 19], but also through their dynamic interactions within coral reef habitats during their early development stages and ontogenetic vertical migrations [1, 20]. The ecological importance of these species includes their role in connecting the zooplankton food web with higher trophic levels, facilitating nutrient cycling within the pelagic environment and, through their extensive diel migrations [25], vertical trophic and biogeochemical connectivity between the upper and mesopelagic layers of the Red Sea.
Parasitism represents an important trophic link and a pervasive ecological strategy where one organism, the parasite, derives benefit from another, the host, often causing harm, while showcasing remarkable adaptations for survival [36]. Mesoparasites, in particular, exhibit specialized adaptations, with a significant portion of their body parts embedded within the host’s tissues [34]. Parasites vary widely in their host specificity, with some being generalists capable of infecting multiple host species, while others are specialists restricted to a single host species [51]. Understanding these parasitic relationships is crucial for comprehending the ecological dynamics of host populations and the role of these, often cryptic, components of food webs.
Among mesoparasites, copepods of the genus Cardiodectes Wilson, 1917, exemplify mesoparasitism within mesopelagic fish communities. Comprising 18 valid species divided into “medusaeus” and “rubosus” groups based on the presence or absence of a defined abdomen [4, 21], Cardiodectes species are parasitic across various teleost families (Table 1). Their life cycle often involves intermediate hosts such as molluscs and mesopelagic fish as definitive hosts [15, 32] – a phenomenon uncommon among fish-parasitic copepods. The evolutionary adaptations and host interactions of these copepods highlight their ecological significance and the complexity of parasitic life cycles.
List of valid species of Cardiodectes Wilson C.B., 1917, including their geographic distribution and host fish species.
Despite their global distribution in oceans, including the Atlantic [3, 9, 32, 45], Indian [8, 13, 24, 28, 33, 35], and Pacific Oceans [4, 5, 14, 15, 21, 26, 29-32, 40, 41, 43, 49, 50, 53, 54, 57], as well as the Mediterranean [6, 7, 16, 22, 54], Arabian [2, 35, 42], and Caribbean Seas [4, 46, 52], Cardiodectes species have notably not been previously documented in the Red Sea. This absence presents a gap in our understanding of the biogeography and diversity of mesoparasitic copepods and the ecology of mesopelagic fish in this unique marine environment.
Here we report the finding, during the Red Sea Decade Expedition (RSDE) 2022, of an undescribed Cardiodectes species parasitizing Vinciguerria mabahiss. This discovery not only fills a geographical gap in the distribution of Cardiodectes, but also presents an opportunity to elucidate its taxonomic identity and phylogenetic relationships within the family Pennellidae. By exploring these findings, this study aims to contribute to our understanding of mesoparasitic adaptations and their ecological implications in the mesopelagic zone of the Red Sea. Additionally, this discovery underscores the importance of ongoing exploration and research in uncovering the hidden biodiversity of mesopelagic ecosystems.
Material and methods
Ethics statement
All fish examined in this study were collected as incidental bycatch during remotely operated vehicle (ROV) operations conducted as part of the Red Sea Decade Expedition. The fish, attracted to the lights of the ROV, were impacted by the thruster motion during coring activities and were already dead at the time of collection; therefore, an Institutional Animal Care and Use Committee (IACUC) permit was not required. This research was conducted in accordance with ethical guidelines and was approved by the Institutional Biosafety and Bioethics Committee (IBEC) of the King Abdullah University of Science and Technology (approval number: 23IBEC055).
Sample collection
Specimens of lightfish were bycatch from sediment core sampling during ROV dives conducted as part of the Red Sea Decade Expedition (RSDE) aboard R/V OceanXplorer between February and June 2022. The ROV entered mesopelagic fish aggregations near the seafloor, and some fish were displaced by thruster activity, settling on the seafloor. These fish were inadvertently trapped in sediment cores and processed onboard. All dives were categorized into different provinces based on the latitude defined by Raitsos and colleagues [37]. Upon collection, inspection of the fish revealed the presence of mesoparasitic pennellid copepods embedded in their bodies. Host specimens were stored at −20 °C onboard and transported to the laboratory at the King Abdullah University of Science and Technology (KAUST). Each fish was measured, weighed, and photographed using a Nikon D7500 digital camera equipped with a 105 mm Micro-Nikkor lens before preservation in 70% ethanol. Incidences of fish-parasite relationships were documented (Supplementary Table 1).
Copepod preparation and examination and specimen deposition
Copepods were removed from the fixed fishes, soaked in lactophenol for approximately half a day, and dissected and examined using the wooden slide method [17]. Drawings were done with the aid of a drawing tube on an Olympus BX53 microscope, and terminology followed [18]. Copepod body parts were measured using an ocular micrometer, and measurements are reported in millimeters as a range. Body and cephalothorax lengths were measured from the tip of rostrum to the posterior tip of trunk and posterolateral lobes, respectively. Cephalothorax width was measured without branching processes. Type specimens examined in this study were deposited in the crustacean collection of the Senckenberg Research Institute (Frankfurt am Main, Germany). The fish specimens (i.e., the hosts) were deposited in the ichthyological collection of the same museum.
DNA extraction, amplification and sequencing
Genomic DNA for molecular analyses was extracted from a female paratype (AA10c) using a DNeasy Blood & Tissue kit (QIAGEN, Hilden, Germany), distinct from the holotype, SMF 63620. Two genes, 18S rDNA and 28S rDNA, were amplified using specific primer pairs: 18SU467F (5′–ATCCAAGGAAGGCAGCAGGC–3′) and 18SL1310R (5′–CTCCACCAACTAAGAACGGC–3′) [47], and 28SF (5′–ACAACTGTGATGCCCTTAG–3′) and 28SR (5′–TGGTCCGTGTTTCAAGACG–3′) [44] in a SimpliAmp™ thermal cycler (Applied Biosystems, Life Technologies, Waltham, MA, USA). PCR reactions were set up in a final volume of 25 μL, containing 1× QIAGEN® Multiplex PCR master mix, 0.2 μM of both forward and reverse primers, 2 μL of genomic DNA, and RNase-free water to adjust the remaining volume. The thermal cycling profiles consisted of an initial denaturation at 95 °C for 15 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 90 s, extension at 72 °C for 90 s, and a final elongation step at 72 °C for 10 min. PCR products were visualized on a 1.5% agarose gel (1× TAE) stained with SYBR Safe (Life Technologies) and electrophoresed at 110 V for 50 min. Amplicons were purified using Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) before bidirectional Sanger sequencing at the KAUST Bioscience Core Lab.
Data analysis
Forward and reverse sequences generated from Sanger sequencing were assembled into contigs using Geneious Prime 2023.1.2 (https://www.geneious.com). Base call quality checks were performed manually prior to merging sequences. Merged sequences were aligned using the Geneious alignment tool before exporting for downstream analysis. Sequences were edited and trimmed to 650 bp (18S) and 541 bp (28S) and subsequently deposited in GenBank (Accession numbers: PQ108883 and PQ108885).
To infer phylogenetic relationships within the Pennellidae family, 18S and 28S reference sequences from 16 pennellid species were downloaded from GenBank and combined with our sequences, using Caligus undulatus as the outgroup (Supplementary Table 2). Sequences were concatenated into a 1,191 bp fragment (18S + 28S), and alignments were exported to MEGA [48] to identify the best substitution model based on the lowest Bayesian Information Criterion (BIC) value. Three phylogenetic trees (18S, 28S, 18S + 28S) were constructed in MrBayes using the mixed + gamma model with default parameters: mcmc ngen = 1,000,000, samplefreq = 500, printfreq = 500, diagnfreq = 5,000 [39]. The resulting .tre files were visualized in FigTree v1.3.1 [38] and edited in Inkscape. Parsimony network analysis was performed for two 18S sequences of Cardiodectes spp. using TCS networks implemented in PopArt [11].
The ecological assessment, including comparisons of standard length, wet weight, and infestation rates across localities, was conducted using RStudio (version 4.3.0). A Mann–Whitney U test was used to compare standard length and wet weight between non-infested and infested host fish. Differences in infestation rates among the five provinces (North, North-Central, Central, South-Central, and South) were analyzed using a Kruskal–Wallis test. The full R script is at available at: https://github.com/limkahkheng/mesopelagic-fish-parasite.
Results
During the Red Sea Decade Expedition 2022, two separate incidences of parasitic infection were observed in mesopelagic fish. Specifically, infestations were documented in one individual of Vinciguerria mabahiss (Fig. 1A) and one individual of Benthosema sp. (Fig. 1B) during separate ROV dives. A total of 158 host-parasite associations was observed among 368 dead specimens of V. mabahiss examined, equating to an infestation prevalence of 43% of the sampled populations. Fifteen fish individuals harbored two copepods, while the remaining fish each hosted a single parasitic copepod. The standard length (SL) of the host fish ranged from 14.89 to 27.75 mm, with an average of 19.97 mm ± 2.03 SD. The wet weight of the host fish varied between 0.03 and 0.17 g, averaging 0.08 g ± 0.03 SD.
 |
Figure 1 ROV footages of (A) lightfish (Vinciguerria mabahiss) and (B) lanternfish (Benthosema sp.) probably infested by mesoparasitic Cardiodectes tofaili n. sp. |
The Mann–Whitney U test indicated no significant difference in standard length between non-infested (N = 204, median = 20.2 mm) and infested (N = 146, median = 19.9 mm) host fish (W = 16420, p = 0.1, Fig. 2A). Similarly, there was no significant difference in wet weight between non-infested and infested host fish (N = 350, median = 0.08 g) (W = 15338, p = 0.63, Fig. 2B). The Kruskal–Wallis test showed no significant differences in the mean ranks of infestation rates among the five provinces (N = 350, H (4) = 1.9757, p = 0.7402).
 |
Figure 2 (A) Boxplot comparing the standard length of host fish infested by copepods versus non-infested. (B) Boxplot comparing the weight of host fish infested by copepods versus non-infested. “ns” denotes non-significant. |
Taxonomy
Order Siphonostomatoida Burmeister, 1835
Family Pennellidae Burmeister, 1835
Genus Cardiodectes Wilson, 1917
Cardiodectes tofaili n. sp. (Figs. 3–5)
urn:lsid:zoobank.org:act:3EB69733-630D-476D-A3FF-AEDF41240785
 |
Figure 3 A specimen of Vinciguerria mabahiss infected by an adult female of Cardiodectes tofaili n. sp. carrying a pair of egg strings. Scale bar 10 mm. |
Type-host: Vinciguerria mabahiss Johnson & Feltes, 1984 (Stomiiformes: Phosichthyidae) (SMF 40114).
Type-locality: CHR0269 (23°47′56.1″N, 38°12′10.3″E), off Yanbu, Red Sea, Saudi Arabia, 645 m depth, 9 May 2022.
Other localities: CHR0197 (21°54′16.2″N, 38°45′28.5″E), off Jeddah, Red Sea, Saudi Arabia, 642 m depth, 22 February 2022; CHR0198 (21°38′25.3″N, 38°54′46.9″E), off Jeddah, Red Sea, Saudi Arabia, 612 m depth, 23 February 2022; CHR0199 (21°25′31.8″N, 39°01′50.2″E), off Jeddah, Red Sea, Saudi Arabia, 454 m depth, 24 February 2022; CHR0201 (20°38′09.2″N, 39°28′03.4″E), off Jeddah, Red Sea, Saudi Arabia, 564 m depth, 26 February 2022.
Attachment site: all type specimens were attached to the specimens of V. mabahiss. The cephalothorax and neck region of the copepod were embedded in the musculature of the host’s trunk, while remaining portion, e.g., trunk and egg strings, exposed outside (Fig. 3).
Type-material: holotype female (SMF 63620) and paratypes: 12 females (SMF 63621-63629).
Prevalence and intensity: one to two copepods in a single host.
Etymology: the species name tofaili is derived from the Arabic word for “parasite,” honoring the nation’s commitment to deep-sea exploration in the Red Sea.
Description (Figs. 4–5; Table 2)
Postmetamorphic adult female. Body (Fig. 4A) 4.71 mm long, comprising cephalothorax, neck region and trunk. Cephalothorax (Figs. 4A–4D) almost as long as wide or slightly longer than wide, 0.86 × 0.85, bearing pairs of nodular and branching anterior processes on anterodistal portion of cephalothorax and pair of anterior spherical lobes, as well as pair of rounded posterolateral lobes extending backwards. Neck region (Figs. 4A–4C) narrow, 0.49 × 0.34, straight: second pedigerous somite swollen, wider than third pedigerous somite; fourth pedigerous somite distinctly segmented and slightly expanded laterally. Trunk (Fig. 4A) slender, ca. five times as long as wide, 3.01 × 0.58, with round posterior margin. Egg strings straight (Fig. 3) and uniseriate, originating at posterolateral genital apertures.
 |
Figure 4 Cardiodectes tofaili n. sp., postmetamorphic adult female, Holotype SMF 63620. A, habitus, dorsal; B, cephalothorax and neck region with digitiform processes removed, ventral; C, same, right side, lateral; D, anterior part of cephalothorax with antennules, antennae and rostrum; E, posterior portion of trunk, ventral; F, same, right side, lateral; G, left antennule, anterior; H, left antenna, anterior; I, left maxilla, anterior. Scale bars: A, 500 μm; B, C, 400 μm; D, 100 μm; E, F, 20 μm; G, 40 μm; H, I, 20 μm. |
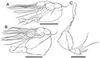 |
Figure 5 Cardiodectes tofaili n. sp., postmetamorphic adult female, Holotype SMF 63620. A, left leg 1 with intercoxal sclerite, posterior; B, left leg 2 with intercoxal sclerite, posterior; C, right leg 3 with intercoxal sclerite, anterior. Scale bars: A–C, 40 μm. |
Armature of legs 1 to 3 of adult female of Cardiodectes tofaili n. sp.
Rostrum, antennules, and antennae situated closely to each other on anterodorsal portion of cephalothorax (Fig. 4D). Rostrum convex with rounded free margin (Figs. 4A, 4E, 4F). Antennule (Fig. 4G) unsegmented, rod-like, bearing at least 5 setae mainly on anterior margin; distal tip bearing at least 8 setae. Antenna (Fig. 4H) 3-segmented, chelate, typical pennellid in form; proximal segment unarmed; middle segment bearing inner pointed projection and pocket; terminal claw with small basal seta on posterior surface. Mouth tube and maxilla situated on anterior part of ventral surface of cephalothorax. Maxilla (Fig. 4I) 2-segmented: proximal segment unarmed; terminal segment separated two parts by constriction at distal four third, bearing fine spinules on basal part and a row of fine spinules on distal claw. Maxilliped absent.
Legs 1 and 2 (Figs. 5A, 5B) biramous, situated on posterior part of cephalothorax (Fig. 4B). Leg 3 (Fig. 5C) uniramous, situated behind anterior swollen on neck. Armature formula of all three legs shown in Table 2. Protopod of leg 3 (Fig. 5C) separated from intercoxal sclerite by long gap.
Variability of female morphology. The morphology of the female paratypes is as in the holotype. The measurements of the paratypes are as follows: body length 3.21–4.46; cephalothorax length 0.65–0.88; cephalothorax width 0.50–0.74; neck length 0.43–0.53; neck width 0.37; trunk length 2.25–2.92; trunk width 0.45–0.60; trunk 4.87 to 4.95 times longer than wide (n = 2).
Remarks. Cardiodectes tofaili n. sp. shares the characteristic of having a trunk without an abdomen with 12 other congeners in the “rubosus” group (see [4, 21]). Although the trunk length of the new species is ca. 5 times longer than wide and only C. krishnai Sebastian, 1968 has such elongate trunk (i.e., more than five times longer than wide), among the 12 congeners (see [2, 4, 21, 26, 28, 42, 46, 49, 50]). The new species is similar to C. krishnai but differs by having the short neck region (i.e., the trunk 5.2 to 6.1 times longer than the neck region) with the second pedigerous somite swollen and the free fourth pedigerous somite (vs. the trunk 3 times longer than the neck region with the second pedigerous somite not swollen and the fourth pedigerous somite not clearly segmented, Sebastian, 1968, fig 1).
Phylogeny of Pennellidae
Analysis of 18S (650 bp), 28S (541 bp) and concatenated 18S + 28S (1,191 bp) sequences from 13–17 species recovered three clades as proposed by [56] (Figs. 6A–6C). Clade-I consisted of Peniculisa and Peniculus spp., while Clade-II consisted of Haemobaphes spp., Phrixocephalus vipereus, Lernaeocera branchialis, Exopenna crimmeni, and Lernaeenicus radiatus. Clade-III consisted of Pseudosarcotretes omorii, Cardiodectes spp. including the Red Sea species, Lernaeenicus spp., and Pennella sp. The Red Sea species (Cardiodectes tofaili n. sp.) is genetically differentiated from the Japanese species (Cardiodectes sp.) by 33 mutational steps based on TCS network analysis of 18S rDNA sequences (Supplementary Figure S1). Unfortunately, the genetic sequence for Cardiodectes krishnai, which is geographically closer and potentially more closely related to Cardiodectes tofaili n. sp. than Cardiodectes sp., is not available, limiting direct comparison and highlighting the need for further genetic characterization of this genus.
 |
Figure 6 Phylogenetic trees of pennellids based on (A) 18S rDNA, (B) 28S rDNA, and (C) concatenated 18S and 28S rDNA sequences, using Caligus undulatus as the outgroup taxa. Node numbers indicate bootstrap values for analyses of posterior probabilities (%) for Bayesian analysis. Scale bars represent nucleotide changes per site. |
Discussion
This study contributes to our understanding of the taxonomy and phylogeny of the Pennellidae family through the discovery of a new species, Cardiodectes tofaili n. sp., parasitizing the endemic Vinciguerria mabahiss in the Red Sea. By incorporating the Red Sea species into our analysis, we conducted a revision of the pennellid phylogenetic tree using 18S rDNA gene sequences from 16 taxa, including both identified species and representative genera. This allowed for a re-evaluation of previous findings by [56, 57]. Our phylogenetic analysis, using Caligus undulatus as the outgroup, clearly distinguishes the Red Sea species from other pennellids. We successfully recovered the three clades proposed by [56], placing Cardiodectes tofaili n. sp. in Clade-III alongside Pseudosarcotretes omorii, Cardiodectes spp., Lernaeenicus spp., and Pennella sp. with a high posterior probability (PP = 100%) (Fig. 6A). The assignment was supported by phylogenetic tress constructed using 28S and concatenated 18S + 28S rDNA gene sequences (Figs. 6B, 6C).
The placement of Cardiodectes tofaili n. sp. within Clade-III suggests a shared evolutionary history with its sister taxa, characterized by adaptations such as the cephalothoracic holdfast, facilitating attachment and survival in the mesopelagic environment [57]. Several species within Clade-III are known to parasitize small, deep-sea fish such as cardinal fish, lanternfish, and lightfish [5, 43, 55, 57]. The relatively large size of these mesoparasitic copepods compared to their small hosts suggests specialized adaptations beyond occupying open spaces like gills and mouths [57]. As proposed by Yumura et al. [56], pennellids may have expanded their host range by adopting mesoparasitic infection strategies. For instance, the cephalothoracic holdfast of Cardiodectes tofaili n. sp. appears to facilitate penetration and anchoring to host muscle tissue, particularly in small, fast-swimming hosts like Vinciguerria mabahiss [23]. Our observation of a similar parasite on a lanternfish Benthosema sp. from ROV footage suggests a potential broader host range. However, definite evidence of taxonomic affinity awaits further investigation, as no parasites were found on collected Benthosema sp. specimens, indicating that although possible, the presence of Cardiodectes seems very rare. Our results suggest that Cardiodectes tofaili n. sp. primarily infects the phosichthyids, whereas Yumura and colleagues reported a Cardiodectes species infecting the grub fish Parapercis sexfasciata in the Pacific Ocean [57]. This discrepancy suggests that Cardiodectes may infect both midwater fish and demersal fish. However, our observation from ROV footage hints a potential, albeit lower, infestation rate of myctophids (Benthosema sp.), with no fish-parasite events detected in 123 dead specimens of Benthosema sp. examined.
Further studies are warranted to explore the ecological implications of these parasitic relationships and their potential impacts on host fitness and population dynamics within the Red Sea ecosystem. In our study, Cardiodectes tofaili n. sp. was found on a wide range of host sizes, ranging from 14.89 to 27.75 mm SL. Importantly, these infestations occurred irrespective of the size of the host, predominantly in the region behind the operculum. Multiple infestations were noted and appeared independent of host sizes. Mesopelagic fish typically live for one to five years [10], during which parasite infections can be transmitted through host behaviors and dietary habits. The life cycle of Cardiodectes typically involves two hosts, with pelagic gastropods acting as intermediate hosts and mesopelagic fish as definitive hosts [32]. Pelagic gastropods, particularly of the genera Clio such as Clio pyramida and C. balantium, as well as the heteropod Carinaria japonica, are known intermediate hosts of Cardiodectes infecting myctophids [32]. Mesopelagic fish in the Red Sea, including V. mabahiss, are known to prey on gastropods during their diel vertical migrations [27], which likely contributes to the observed infestation patterns of Cardiodectes tofaili n. sp. Further investigations are needed to verify the species identity of intermediate hosts of Cardiodectes in the Red Sea.
Acknowledgments
We sincerely thank the National Center for Wildlife (NCW) for inviting us to participate in the Red Sea Decade Expedition (2022). We are grateful to Ms. Jennifer Thompson for her assistance in collecting the fish specimens during the expedition. Special thanks to Dr. Arthur Anker for recognizing the copepod family and suggesting the species epithet. Our sincere thanks go to Dr. Amal Bajaffer for verifying the species name in Arabic. This collaboration was facilitated by Dr. Matthew Tietbohl. The Red Sea Decade Expedition was funded by the National Center for Wildlife (NCW, Kingdom of Saudi Arabia). King Abdullah University of Science and Technology (KAUST) also supported this research through baseline funding awarded to CMD (BAS/1/1071-01-01); the Japan Society for Promotion of Science (JSPS) KAKENHI Grant Numbers JP23K27232 and JP24K09575; and the “Establishment of Glocal Research and Education Network in the Amami Islands” project of Kagoshima University.
Conflicts of interest
All authors declare that they have no conflict of interest.
Supplementary materials
 |
Supplementary Figure S1. Statistical parsimony network of Cardiodectes based on 18S rDNA sequences. The network illustrates the genetic differentiation between the newly described Red Sea species, Cardiodectes tofaili n. sp., and the Japanese species, Cardiodectes sp., with the latter being the only sequence available from this genus in GenBank (LC777455). Each circle represents a haplotype, with species distinguished by a different pattern. |
Supplementary Table 1. Metadata on the collection of the host Vinciguerria mabahiss infected by the mesoparasitic Cardiodectes tofaili sp. nov., during the Red Sea Decade Expedition (RSDE) 2022. Data are displayed for complete individuals with morphometric measurements. Twelve incomplete host fish infected with copepods are not shown as measurements could not be obtained. Access here
Supplementary Table 2. 18S and 28S sequences of pennellids and caligid (Caligus undulatus) used in current study, with GenBank accession numbers. Access here
References
- Abu El-Regal MA, Ditty JG. 2023. Development and seasonal variations of the larvae of three mesopelagic fishes near coral reefs in the Red Sea. Fishes, 8(10), 500. [Google Scholar]
- Aneesh PT, Helna AK, Kumar AB, Maran BAV. 2023. A new species of Cardiodectes Wilson C.B., 1917 (Copepoda: Siphonostomatoida: Pennellidae) from Spinyjaw greeneye, Chlorophthalmus corniger Alcock, 1894 off the Indian Ocean. Zootaxa, 5369(2), 277–291. [Google Scholar]
- Barnard KH. 1955. South African parasitic Copepoda. Annals of the South African Museum [Annale van die Suid-Afrikaanse Museum], 41, 223–312. [Google Scholar]
- Bellwood DR. 1981. Two new species of Cardiodectes Wilson (Copepoda: Siphonostomatoida). Systematic Parasitology, 2, 149–156. [Google Scholar]
- Boxshall GA. 2000. Parasitic copepods (Copepoda: Siphonostomatoida) from deep-sea and mid-water fishes. Systematic Parasitology, 47(3), 173–181. [Google Scholar]
- Brian A. 1906. Copepodi parassiti dei pesci d’Italia (con XXI tavole). Genova: Stab. Tipo-Litografico R. Istituto Sordomuti. [Google Scholar]
- Brian A. 1912. Copépodes parasites de poissons et des échinides provenant des campagnes scientifiques de S.A.S. le prince Albert Ier de Monaco, 1886-1910. p. 1–58. [Google Scholar]
- Brian A, Gray P. 1928. Morphologie externe et interne d’un nouveau copépode parasite Cardiodectes anchorellae n. sp., trouvé à Madras. Bollettino dei Musei di Zoologia e Anatomia Comparata della R. Università di Genova, 8(26), 1–10. [Google Scholar]
- Capart A. 1953. Quelques Copépodes parasites de poissons marins de la région de Dakar. Bulletin de l’Institut Français d’Afrique Noire, 15, 647–671. [Google Scholar]
- Catul V, Gauns M, Karuppasamy P. 2011. A review on mesopelagic fishes belonging to family Myctophidae. Reviews in Fish Biology and Fisheries, 21, 339–354. [Google Scholar]
- Clement M, Snell Q, Walke P, Posada D, Crandall K. 2002. TCS: Estimating gene genealogies, in: Parallel and Distributed Processing Symposium, International. IEEE Computer Society. [Google Scholar]
- Gjøsæter J, Kawaguchi K. 1980. A review of the world resources of mesopelagic fish. FAO Fisheries Technical Paper No. 193, Rome, Italy. p. 1–151. [Google Scholar]
- Gnanamuthu CP. 1951. Studies on a lernacid copepod, Cardiodectes anchorellae Brian and Gray. Proceedings of the Zoological Society of London, 121(2), 237–252. [Google Scholar]
- Hirose E, Uyeno D. 2016. Regional differentiation of the cuticular surface structure in the mesoparasitic copepod Cardiodectes shini (Siphonostomatoida: Pennellidae) on a pygmy goby. Invertebrate Survival Journal, 13, 134–139. [Google Scholar]
- Ho JS. 1966. Larval stages of Cardiodectes sp. (Caligoida: Lernaeoceriformes), a copepod parasitic on fishes. Bulletin of Marine Science, 16(2), 159–199. [Google Scholar]
- Hogans WE. 2017. Cardiodectes medusaeus (Copepoda: Pennellidae) a synonym of Cardiodectes bellottii, a parasite of mid-water fishes in the North Atlantic Ocean and Mediterranean Sea. Proceedings of the Biological Society of Washington, 130(1), 250–255. [Google Scholar]
- Humes AG, Gooding RU. 1964. A method for studying the external anatomy of copepods. Crustaceana, 6(3), 238–240. [Google Scholar]
- Huys R, Boxshall GA. 1991. Copepod evolution, Vol. 159. London: The Ray Society. 468 pp. [Google Scholar]
- Irigoien X, Klevjer TA, Rostad A, Martinez U, Boyra G, Acuña JL, Bode A, Echevarria F, Gonzalez-Gordillo JI, Hernandez-Leon S, Agusti S, Aksnes DL, Duarte CM, Kaartvedt S. 2014. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nature Communications, 5(1), 3271. [Google Scholar]
- Isari S, Pearman JK, Casas L, Michell CT, Curdia J, Berumen ML, Irigoien X. 2017. Exploring the larval fish community of the central Red Sea with an integrated morphological and molecular approach. Plos One, 12(8), e0182503. [Google Scholar]
- Izawa K. 1970. A parasitic copepod, Cardiodectes rotundicaudatus n. sp., (Caligoida: Lernaeidae) obtained from a deep-sea goby in Japan. Annotationes Zoologicae Japonenses, 43(4), 219–224. [Google Scholar]
- Jungersen HFE. 1911. On a new gymnoblastic hydroid (Ichthyocodium sarcotretis) epizoic on a new parasitic copepod (Sarcotretes scopeli) infesting Scopelus glacialis Rhdt. Videnskabelige meddelelser fra den Naturhistoriske forening i Kjöbenhavn 64, 1–33. [Google Scholar]
- Kaartvedt S, Christiansen S, Rostad A, Aksnes DL. 2022. Mesopelagic fishes in a hurry at low latitudes. Marine Ecology Progress Series, 694, 149–156. [Google Scholar]
- Kirtisinghe P. 1964. A review of the parasitic copepods of fish recorded from Ceylon with description of additional forms. Bulletin of the Fisheries Research Station, Ceylon, 17, 45–132. [Google Scholar]
- Klevjer TA, Irigoien X, Rostad A, Fraile-Nuez E, Benítez-Barrios VM, Kaartvedt S. 2016. Large scale patterns in vertical distribution and behaviour of mesopelagic scattering layers. Scientific Reports, 6(1), 19873. [Google Scholar]
- Leigh-Sharpe WH. 1934. The Copepoda of the Siboga Expedition. Part II. Commensal and parasitic Copepoda. Siboga Expeditie, Monograph, Leiden, 29b, 1–43. [Google Scholar]
- Lim KK, Angulo-Preckler C, Hempel CA, Qurban MA, Pieribone VA, Duarte CM. 2025. Benthic feeding and diet partitioning in Red Sea mesopelagic fish resolved through DNA metabarcoding and ROV footage. Ecology and Evolution, 15(3), e71091. [Google Scholar]
- Markewitsch A. 1936. Cardiodectes hardenbergi, ein neuer parasitischer copepode aus der Java See. Treubia Deel, 15(4), 407–411. [Google Scholar]
- Moser M, Taylor S. Effects of the copepod Cardiodectes medusaeus on the laternfish Stenobrachius leucopsarus with notes on hypercastration by the hydroid Hydrichthys sp., Canadian Journal of Zoology, 1978) 56(11), 2372–2376. [Google Scholar]
- Noble ER, Collard SB. 1970. The parasites of midwater fishes, in: Symposium of diseases of fishes and shellfishes, Snieszko SF, Editor. Washington, DC: American Fisheries Society, pp. 57–69. [Google Scholar]
- Paxton JR. 1967. Biological note on southern California lanternfishes (family Myctophidae). California Fish and Game, 53(3), 214–217. [Google Scholar]
- Perkins PS. 1983. The life history of Cardiodectes medusaeus (Wilson), a copepod parasite of lanternfishes (Myctophidae). Journal of Crustacean Biology, 3(1), 70–87. [Google Scholar]
- Pesta O. 1933. Zoogeographische Berichte über Crustaceen. Zoologischer Anzeiger, 104, 274–282. [Google Scholar]
- Piasecki W, Barcikowska D, Panicz R, Eljasik P, Kochmanski P. 2022. First step towards understanding the specific identity of fish muscle parasites of the genus Sarcotaces (Copepoda: Philichthyidae)-New species and first molecular ID in the genus. International Journal for Parasitology-Parasites and Wildlife, 18, 33–44. [Google Scholar]
- Pillai KN. 1985. The Fauna of India, in: Copepod Parasites of Marine Fishes. Zoological Society of India, Calcutta, p. 900. [Google Scholar]
- Poulin R. 2007. Are there general laws in parasite ecology? Parasitology, 134, 763–776. [Google Scholar]
- Raitsos DE, Brewin RJW, Zhan P, Dreano D, Pradhan Y, Nanninga GB, Hoteit I. 2017. Sensing coral reef connectivity pathways from space. Scientific Reports, 7, 9338. [Google Scholar]
- Rambaut A. 2010. FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/. [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3), 539–542. [CrossRef] [PubMed] [Google Scholar]
- Sakuma KM, Ralston S, Lenarz WH, Embury M. 1999. Effects of the parasitic copepod Cardiodectes medusaeus on the lanternfishes Diaphus theta and Tarletonbeania crenularis off central California. Environmental Biology of Fishes, 55(4), 423–430. [Google Scholar]
- Schuurmans Stekhoven Jr JH. 1937. Crustacea Parasitica, in: Résultats Scientifiques des Croisières du Navire-École Belge (Mercator). Belgium: Verhandelingen van het Koninklijk Natuurhistorisch Museum, pp. 11–26. [Google Scholar]
- Sebastian MJ. 1968. Cardiodectes krishnai, a new species of lernaeid copepod from the fish, Vinciguerria lucetia (Garman). Crustaceana, Supplement (1), 136–140. [Google Scholar]
- Shiino S. 1958. Copepods parasitic on Japanese fishes. 17. Lernaeidae. Report of the Faculty of Fisheries, Prefectural University of Mie, 3, 75–100. [Google Scholar]
- Song Y, Wang GT, Yao WJ, Gao Q, Nie P. 2008. Phylogeny of freshwater parasitic copepods in the Ergasilidae (Copepoda: Poecilostomatoida) based on 18S and 28S rDNA sequences. Parasitology Research, 102(2), 299–306. [Google Scholar]
- Stebbing TRR. 1917. South African Crustacea (Part IX of S.A. Crustacea, for the Marine Investigations in South Africa). Annals of the South African Museum. Annale van die Suid-Afrikaanse Museum, 17, 23–46. [Google Scholar]
- Suárez-Morales E, Vásquez-Yeomans L, Vidotto E. 2022. A new Cardiodectes Wilson, 1917 (Hexanauplia: Copepoda: Siphonostomatoida) parasitic on a scarid teleost (Perciformes: Scaridae) from Roatan Island, Central America. Systematic Parasitology, 99(6), 707–714. [Google Scholar]
- Suzuki N, Murakami K, Takeyama H, Chow S. 2006. Molecular attempt to identify prey organisms of lobster phyllosoma larvae. Fisheries Science, 72(2), 342–349. [Google Scholar]
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38(7), 3022–3027. [CrossRef] [PubMed] [Google Scholar]
- Uyeno D. 2013. Two new species of Cardiodectes Wilson, 1917 (Copepoda: Siphonostomatoida: Pennellidae) from gobiid fishes (Actinopterygii: Perciformes) in the western Pacific Ocean. Zootaxa, 3664(3), 301–311. [Google Scholar]
- Uyeno D, Nagasawa K. 2010. Three new species of the family Pennellidae (Copepoda: Siphonostomatoida) from gobiid fishes (Actinopterygii: Perciformes) in coastal waters of the western Pacific Ocean. Zootaxa, 2687, 29–44. [Google Scholar]
- Walker JG, Hurford A, Cable J, Ellison AR, Price SJ, Cressler CE. 2017. Host allometry influences the evolution of parasite host-generalism: theory and meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1719), 20160089. [Google Scholar]
- Williams EH Jr., Bunkley‐Williams L, Sanner CJ. 1994. New host and locality records for copepod and isopod parasites of Colombian marine fishes. Journal of Aquatic Animal Health, 6(4), 362–364. [Google Scholar]
- Wilson CB. 1908. North American parasitic copepods: a list of those found upon the fishes of the Pacific coast, with descriptions of new genera and species. Proceedings of the United States National Museum, 35, 431–481. [Google Scholar]
- Wilson CB. 1917. North American parasitic copepods belonging to the Lernaeidae with a revision of the entire family. Proceedings of the United States National Museum, 53(2194), 1–150. [Google Scholar]
- Yamaguti S, Utinomi H. 1953. Lernaeenicus quadrilobatus n. sp. (Copepoda, Lernaeidae) parasitic on the lantern-fish Diaphus coeruleus. Publications of the Seto Marine Biological Laboratory, 3(1), 51–53. [Google Scholar]
- Yumura N, Adachi K, Nitta M, Kondo Y, Komeda S, Wakabayashi K, Fukuchi J, Boxshall GA, Ohtsuka S. 2022. Exploring evolutionary trends within the Pennellidae (Copepoda: Siphonostomatoida) using molecular data. Systematic Parasitology, 99(4), 477–489. [Google Scholar]
- Yumura N, Nishikawa J, Ohtsuka S. 2024. A new species and a new genus of the family Pennellidae (Copepoda: Siphonostomatoida) parasitic on North Pacific lightfish Maurolicus japonicus (Actinopterygii: Stomiiformes: Sternoptychidae) collected from Suruga Bay, Japan. Species Diversity, 29(1), 81–90. [Google Scholar]
Cite this article as: Lim KK, Angulo-Preckler C, Rabaoui LJ, Qurban MA, Pieribone VA, Duarte CM & Uyeno D. 2025. Discovery of new species of mesoparasitic pennellid (Copepoda: Siphonostomatoida) from the endemic mesopelagic lightfish Vinciguerria mabahiss in the Red Sea. Parasite 32, 43. https://doi.org/10.1051/parasite/2025038.
All Tables
List of valid species of Cardiodectes Wilson C.B., 1917, including their geographic distribution and host fish species.
All Figures
 |
Figure 1 ROV footages of (A) lightfish (Vinciguerria mabahiss) and (B) lanternfish (Benthosema sp.) probably infested by mesoparasitic Cardiodectes tofaili n. sp. |
| In the text | |
 |
Figure 2 (A) Boxplot comparing the standard length of host fish infested by copepods versus non-infested. (B) Boxplot comparing the weight of host fish infested by copepods versus non-infested. “ns” denotes non-significant. |
| In the text | |
 |
Figure 3 A specimen of Vinciguerria mabahiss infected by an adult female of Cardiodectes tofaili n. sp. carrying a pair of egg strings. Scale bar 10 mm. |
| In the text | |
 |
Figure 4 Cardiodectes tofaili n. sp., postmetamorphic adult female, Holotype SMF 63620. A, habitus, dorsal; B, cephalothorax and neck region with digitiform processes removed, ventral; C, same, right side, lateral; D, anterior part of cephalothorax with antennules, antennae and rostrum; E, posterior portion of trunk, ventral; F, same, right side, lateral; G, left antennule, anterior; H, left antenna, anterior; I, left maxilla, anterior. Scale bars: A, 500 μm; B, C, 400 μm; D, 100 μm; E, F, 20 μm; G, 40 μm; H, I, 20 μm. |
| In the text | |
 |
Figure 5 Cardiodectes tofaili n. sp., postmetamorphic adult female, Holotype SMF 63620. A, left leg 1 with intercoxal sclerite, posterior; B, left leg 2 with intercoxal sclerite, posterior; C, right leg 3 with intercoxal sclerite, anterior. Scale bars: A–C, 40 μm. |
| In the text | |
 |
Figure 6 Phylogenetic trees of pennellids based on (A) 18S rDNA, (B) 28S rDNA, and (C) concatenated 18S and 28S rDNA sequences, using Caligus undulatus as the outgroup taxa. Node numbers indicate bootstrap values for analyses of posterior probabilities (%) for Bayesian analysis. Scale bars represent nucleotide changes per site. |
| In the text | |
 |
Supplementary Figure S1. Statistical parsimony network of Cardiodectes based on 18S rDNA sequences. The network illustrates the genetic differentiation between the newly described Red Sea species, Cardiodectes tofaili n. sp., and the Japanese species, Cardiodectes sp., with the latter being the only sequence available from this genus in GenBank (LC777455). Each circle represents a haplotype, with species distinguished by a different pattern. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


