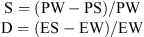| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 17 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/parasite/2024020 | |
| Published online | 26 March 2024 | |
Research Article
Sterile Insect Technique (SIT) field trial targeting the suppression of Aedes albopictus in Greece
Essai sur le terrain de la Technique de l’Insecte Stérile (TIS) ciblant la suppression d’Aedes albopictus en Grèce
1
Scientific Directorate of Entomology and Agricultural Zoology, Benaki Phytopathological Institute, 14561 Kifissia, Greece
2
Centro Agricoltura Ambiente “G. Nicoli”, 40014 Crevalcore, Italy
3
Insect Pest Control Laboratory, Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture, Seibersdorf, A-2444 Vienna, Austria
4
Department of Agriculture, Crop Production and Rural Environment, University of Thessaly, 38446 Magnisias, Greece
5
Laboratory of Applied Zoology and Parasitology (Entomology), School of Agriculture, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
6
ASTRE, CIRAD, 34398 Montpellier, France
7
ASTRE, Cirad, INRAE, Univ. Montpellier, Plateforme Technologique CYROI, Sainte-Clotilde, La Réunion, France
* Corresponding author: a.michaelakis@bpi.gr
Received:
23
November
2023
Accepted:
6
March
2024
The sterile insect technique (SIT) involves releasing large numbers of sterile males to outcompete wild males in mating with females, leading to a decline in pest populations. In the current study, we conducted a suppression trial in Greece against the invasive dengue vector mosquito Aedes albopictus (Skuse) through the weekly release of sterile males for 22 weeks from June to September 2019. Our approach included the long-distance transport of sterile mosquitoes, and their release at a density of 2,547 ± 159 sterile males per hectare per week as part of an area-wide integrated pest management strategy (AW-IPM). The repeated releases of sterile males resulted in a gradual reduction in egg density, reaching 78% from mid-June to early September. This reduction remained between 70% and 78% for four weeks after the end of the releases. Additionally, in the SIT intervention area, the ovitrap index, representing the percentage of traps containing eggs, remained lower throughout the trial than in the control area. This trial represents a significant advance in the field of mosquito control, as it explores the viability and efficacy of producing and transporting sterile males from a distant facility to the release area. Our results provide valuable insights for future SIT programmes targeting Ae. Albopictus, and the methodology we employed can serve as a starting point for developing more refined and effective release protocols, including the transportation of sterile males over long distances from production units to intervention areas.
Résumé
La technique de l’insecte stérile (TIS) consiste à libérer un grand nombre de mâles stériles pour supplanter les mâles sauvages lors de l’accouplement avec les femelles, entraînant ainsi un déclin des populations de nuisibles. Dans la présente étude, nous avons mené un essai de suppression en Grèce contre le moustique vecteur invasif de la dengue, Aedes albopictus (Skuse), par le biais de la libération hebdomadaire de mâles stériles pendant 22 semaines de juin à septembre 2019. Notre approche comprenait le transport sur de longues distances de moustiques stériles, et leur lâcher à une densité de 2 547 ± 159 mâles stériles par hectare et par semaine dans le cadre d’une stratégie de lutte intégrée contre les nuisibles à l’échelle de la zone (AW-IPM). Les lâchers répétés de mâles stériles ont entraîné une réduction progressive de la densité des œufs, atteignant 78 % de la mi-juin au début septembre. Cette réduction est restée entre 70 % et 78 % pendant quatre semaines après la fin des lâchers. De plus, dans la zone d’intervention de la TIS, l’indice d’oviposition, représentant le pourcentage de pièges contenant des œufs, est resté plus faible que dans la zone témoin tout au long de l’essai. Cet essai représente une avancée significative dans le domaine de la lutte contre les moustiques, car il explore la viabilité et l’efficacité de la production et du transport de mâles stériles depuis une installation éloignée vers la zone de lâcher. Nos résultats fournissent des informations précieuses pour les futurs programmes de TIS ciblant Ae. albopictus et la méthodologie que nous avons utilisée pourra servir de point de départ pour développer des protocoles de libération plus raffinés et plus efficaces, y compris le transport de mâles stériles sur de longues distances depuis les unités de production jusqu’aux zones d’intervention.
Key words: Egg density / Egg hatching rates / Sterile male insect transportation / Mosquito borne diseases / Public health
© G. Balatsos et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Aedes invasive mosquito species (AIM) have been recorded in many European and Mediterranean countries since the first detection of Aedes albopictus (Skuse), in the 1970s in Albania [10]. AIM mosquito species can enter and establish permanent populations in new areas with favourable environmental and climatic conditions. This phenomenon is being further exacerbated by climate change [16]. Consequently, new public health risks are faced, including the increasing incidence of mosquito-borne diseases (MBDs) such as chikungunya and dengue, which are currently emerging in different European countries [19, 20].
The Asian tiger mosquito (Ae. albopictus) is the main AIM species in Europe, already causing public health problems as well as intense nuisance [10]. Due to its aggressive and day biting behaviour, it is affecting public perception regarding the effectiveness of mosquito control programmes. The establishment of Ae. albopictus in urban and semi-urban areas challenges the existing mosquito control programmes that are coordinated by local authorities and executed mainly by various private entities [1].
The management plan to control Ae. albopictus is thus a complex system that includes coordinated actions to control its populations. Conventional control programmes applied successfully against marshland mosquitoes are less effective in controlling the population of Ae. albopictus in urban areas due to its unique bioecology, e.g., cryptic breeding behaviour, daily biting behaviour and the dispersion of breeding sites [10, 26]. Cases of resistance to insecticides in Ae. albopictus populations in certain European countries has been reported [32], whereas there are limited options available for larvicidal use. Therefore, there is a need to implement alternatives to chemically based vector control methods and strategies, such as the sterile insect technique (SIT) and citizen science [24, 31].
The SIT method is based on the release of large numbers of sterile males to outcompete wild males for mating with females. The SIT involves three key steps: production of target species in large numbers under controlled environments; male sterilisation using ionising radiation and systematic release of sterile individuals within the target area [25]. This method relies on the sterile males’ ability to mate with wild females, thereby decreasing the overall reproductive success of the population. Its successful application has led to improvements in pest control, minimising the need for chemical interventions and reducing associated environmental risks [18]. This is also in accordance with the EU policy as set out in EU Regulation 528/2012 aiming to reduce the use of biocides.
The efficacy of SIT can be enhanced through further improvements, and it can be effectively integrated with other vector control strategies, including source reduction [31]. Source reduction refers to the elimination of breeding habitats, aiming to minimise the availability of suitable sites for mosquito reproduction. Despite the extensive success of the SIT in controlling agricultural insect pests, its implementation for mosquito vectors of human diseases is currently at an early stage of development. Pilot trials are being conducted to assess the viability and efficacy of SIT as a method for controlling mosquito populations, specifically targeting species belonging to the Aedes spp.
In 2019, a suppression trial took place in Vravrona located in the Attica Region of Greece. Prior to this trial, in the 2018 summer period, a door-to-door strategy had previously been implemented, raising public awareness through knowledge, attitude, and practice (KAP) surveys aiming to reduce breeding sites both in private and public areas [34]. Additionally, a pilot release of sterile males had been conducted at the end of the previous mosquito season in 2018 [2]. The suppression trial was conducted throughout the mosquito season and the primary goal was to evaluate the efficacy of the SIT in suppressing Ae. albopictus populations in Greece, within the context of an area wide (AW) approach. Additionally, the study aimed to explore the long-distance transfer of sterile male mosquitoes. Therefore, all sterile males were exclusively produced at the Centro Agricoltura Ambiente “G. Nicoli” (CAA, Italy) facility and transported to the specified release plot [2, 29]. To our knowledge, this study is the first attempt to use the SIT for mosquito suppression, which is based on long distance production of sterile males.
Materials and methods
Description of plots
The suppression trial took place in Vravrona, Markopoulo municipality, Attica Region (East Regional Unit) of Greece located east of Athens (SIT plot) (Fig. 1 and Table 1). The total focal area of the Vravrona site was almost 10 ha. To the north, the area is surrounded by the sea, while to the south, west, and east, the nearest urban areas are approximately 1.5 km away. Therefore, it was isolated from other urban areas since the focal area was surrounded by high hills covered by montane forests. Based on the previous trial, there was no significant difference between the control and SIT plot [2]. To compare the efficacy of SIT in the current study with that of the previous study, we established two control plots (5 ha each) in semi-urban areas close to the release plot. Control 1 is located 1 km east of the SIT plot, and control 2, more than 4 km north of the SIT area (Fig. 1 and Table 1). Moreover, in collaboration with the Municipality of Markopoulo, no chemical treatments were implemented during the trial in public areas falling under the municipality’s authority.
 |
Figure 1 Geographic position of the SIT plots and the respective control plots C1 and C2 (A) and distribution of releasing points within the SIT plot (B). |
Description of the study plot and the respective control plots including the number of ovitraps deployed to assess the efficacy of the SIT trial.
Sterile male production and handling
All sterile Ae. albopictus males (Greek strain) were produced at the CAA mass rearing facility using methods described in Bellini et al. [8] and Malfacini et al. [28]. The mosquito colony used for the current suppression trial originated from eggs collected in Vravrona in 2017, 2018 and 2019 [2]. Mosquito larvae were mass reared according to the IAEA protocols [3, 27]. Males were irradiated at the pupal stage with a dose of 35 Gy using an IBL 437 irradiator (CIS Bio International, Saclay, France) equipped with a Cs-137 linear source. Based on this protocol, this irradiation level strikes the optimal balance between sterility and the performance of sterile males. Furthermore, the age of the male mosquito pupa at the time of irradiation (calculated from pupation to the irradiation moment) exerts a substantial influence on both the achieved sterility level and the quality of the resulting adults [4].
Transportation of sterile males and release
Male mosquitoes were chilled in a large cooling cabinet (8 ± 1 °C, 85 ± 5% RH) for approximately one hour before packaging. The cold-shock anaesthetised sterile males were then transferred to small plastic cylindrical containers (plastic box 5 cm diameter, 5 cm height, 80 cc capacity) placed and sealed with tape inside a larger plastic container (PP plastic, 20 × 15 × 6 h cm, 1,800 cc capacity). Up to three of these plastic containers were stacked vertically and packed inside a polystyrene container with adequate quantity of phase-changing materials (PCM) to maintain a temperature around 12 °C and delivered by express courier service from the production facility (Centro Agricoltura Ambiente “G. Nicoli” – CAA) to Athens as described in Mastronikolos [29]. Upon arrival, sterile males were immediately transferred to the release plot.
Cup shaped paper containers (paper box, diameter of upper base 12 cm and lower base 11 cm, 8 cm height) were used to release sterile males. Each paper container (release cup) was previously hardened on its inner surface to allow the sterile male mosquitoes to rest. Each container was hung by rope on one of the 30 predefined permanent release points (e.g., tree branches, fences, etc.) (Fig. 1). Sterile males were released on a weekly basis for 22 weeks (from 03 May to 04 October 2019).
All releases were conducted approximately 1.5 h after receipt of shipments from Italy, in the 30 predefined permanent stations (release points) established in the SIT plot. Before releasing them, the anesthetised sterile males were transferred into the release cup, stoppered with mesh, which contained a 10% sugar solution, to regain their activity. After one hour, all paper containers (release cups) were distributed to the release sites. Some vaseline (Vaseline Original Pure Petroleum Jelly, Unilever, London, UK) was applied on the hanging rope to prevent possible access of predatory insects (e.g., ants). The release time lasted ca. 30 min (Video 1 available in Supplementary material). Sterile males that died during the release process or were unable to fly from the paper containers were collected and counted.
Entomological monitoring to assess the impact of SIT application
To assess the impact of sterile male releases on the population dynamics of Ae. albopictus, we established a network of ovitraps, each consisting of a 1.5 L black plastic container accompanied by a wooden strip measuring 150 × 18 × 1.6 mm. In all plots, including both the SIT and control, we deployed three ovitraps per hectare, totalling 60 ovitraps (Table 1). We complied with the standard operational procedures for ovitrap field management, as outlined in Annexes 1 and 2 of Bellini et al. [10]. Throughout the year 2019, all ovitraps underwent weekly inspections, starting from 01 January and concluding on 31 December. Table 2 provides a comprehensive summary of weekly releases, including the quantity of mosquitoes delivered per week, the associated mortality rate, and the estimated density of sterile males released per hectare.
Descriptive data of the 22 weekly releases of sterile males carried out in Greece in 2019.
Weather and environmental parameters
In each plot (SIT and controls), the temperature and RH was recorded by a temperature-humidity logger with an internal sensor (type Ebro EBI 20 TH1, Xylem Analytics Germany Sales GmbH & Co, Weilheim, Germany). These records are provided in Figure 2.
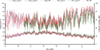 |
Figure 2 Temperature and Relative Humidity (RH) data at the SIT and control plots [SIT: 65.030 ± 0.185 RH and 23.414 ± 0.062 °C], [Control 1 (C1): 63.796 ± 0.178 RH and 23.315 ± 0.056 °C], [Control 2 (C2): 57.887 ± 0.205 RH and 25.175 ± 0.067 °C]. |
Assessment of egg hatching rates
The oviposition substrates were collected weekly and managed carefully to protect eggs from desiccation during transportation to the laboratory (Annexes 1 and 2 in Bellini et al. [10]). At the laboratory, the oviposition substrates were left at 25 ± 1 °C, 80% RH, 14:10 L:D for one day and then placed in a container with a saturated solution of potassium sulphate (K2SO4) to complete embryonation for a minimum of six days. A protocol described in Bellini et al. [8, 9] was adopted for egg hatching. The steps involved in egg collection and storage of the oviposition substrate are crucial in assessing the hatching of eggs. In Table 3, we describe each step, including important information and notes that should be taken into account. This comprehensive summary aims to assist in achieving successful egg hatching outcomes.
Description of steps to be followed to assess egg hatch.
Statistical analyses
The induced sterility (S) and decreased egg density (D) were calculated using the equations:
where ES and EW are the mean number of eggs per ovitrap per week in SIT and control plot, while PS and PW are the percentage of hatched eggs, in the SIT and in the control plot, respectively [7].
Generalised Poisson linear mixed models (GLMM) were used to assess the effect of SIT on egg density and binomial generalised linear mixed models were used to test the effect of treatment on egg sterility (hatch rate). Treatment, duration of treatment (weeks) and their first order interaction were used as fixed effects and trap numbers and dates were used as random effects. The full models were compared to simpler models using the second-order Akaike Information Criterion [15, 23]. The analysis was conducted until 25 October 2019 after which the oviposition activity and hatch rates dropped in all plots due to diapause.
Results
Transportation of sterile males and release
Mortality of sterile males during transport from Italy to the release point in Greece was on average 16.19 ± 1.62%. The duration of transportation from the CAA mass rearing facility in Italy to the field release plots in Vravrona was approximately 21 h (Table 2).
Release of sterile males
For 22 weeks, 03 May to 04 October (2019), 662,000 sterile males were released. The mean release density per hectare per week was 2,547 (±159.3) sterile males, with an average female contamination of 0.76 ± 0.12% (Table 2).
Assessment of egg hatching rate
Egg hatching rates were higher overall in control 2 plot than in control 1 and SIT plots (p < 10−3, Table 4). Hatch rates were constant over time in control 2 (p = 0.50), increased in control 1 (p < 0.001) and decreased in the SIT plot (p < 0.01) (Fig. 3). The seasonal average of the rate of induced sterility (S) from 03 May to 04 October 2019 was 48.87% (±13.57) with a minimum of 15.80% during the period 05–12 July 2019 (high temperatures; 34 °C) and a maximum of 77.95% from 30 August to 5 September (Fig. 4).
 |
Figure 3 Hatching rates at SIT and control plots. The hatching rates of collected eggs (oviposition substrates) from 60 ovitraps were assessed weekly. |
 |
Figure 4 Seasonal patterns of induced sterility and egg reduction at the SIT plot as compared with the control area (right Y axis). The grey bars indicate the number of sterile males released per hectare (ha) per week from 03 May to 04 October (2019) at the SIT plot (left Y axis). |
Fixed-effects coefficients of a mixed-effect binomial model of the impact of treatment, time and their interaction on the hatch rate of eggs of Aedes albopictus (1,426 observations, 60 traps, 24 collection dates, Control 2 site used as a reference).
Egg density evaluation
The average egg density recorded in the SIT plot was similar to that of control 2 (Table 5, p = 0.82) but initially higher than in control 1 (p < 0.01) at the beginning of the trial. Egg density increased over time in controls 2 and 1 (p < 0.01), whereas it decreased in the SIT plot (p < 0.01), demonstrating significant suppression (Fig. 5). During the period from mid-June to early September, the egg reduction rate exhibited a significant increase, reaching 78%, and this elevated rate persisted within the range of 70–78% for up to 4 weeks following conclusion of the releases (Fig. 4).
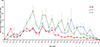 |
Figure 5 The seasonal trend of egg density (mean ± SE) collected at SIT and control plots C1 and C2. |
Fixed-effects coefficients of a mixed-effect Poisson model of the impact of treatment, time and their interaction on the density of eggs of Aedes albopictus (1,426 observations, 60 traps, 24 collection dates, Control 2 site used as a reference).
Oviposition positivity index (OPI)
The percentage of OPI varied at similar rates from May until mid-August among the three plots, but declined substantially from this point to the end of the season in the SIT compared to two control plots (Fig. 6). Also, it is noteworthy that the POI in the SIT plot did not reach 100% positivity at any point during the trial.
 |
Figure 6 Weekly distribution of oviposition positivity index (OPI) at the release (SIT) and control plots C1 and C2 from 03 May to 29 November (2019). |
Discussion
In 2018, toward the end of the mosquito season, a door-to-door strategy was implemented in Vravrona (target area) to reduce breeding sites in both private and public areas before the application of the pilot release of sterile males [2, 34]. The results from the suppression trial in 2019 highlighted the efficacy of the SIT, as part of an AW based approach, even in a small plot and in a one-year trial. The integration of SIT with complementary interventions has demonstrated the potential for achieving a substantial reduction in Ae. albopictus populations. This approach not only mitigates mosquito nuisance, but it also holds promise in significantly diminishing the incidence and transmission of vector-borne diseases [5, 34, 37]. Several countries worldwide have undertaken pilot projects and field trials involving SIT to reduce populations of Ae. albopictus using local production facilities for the sterile males such as Italy [9], Spain [35], Mexico [11] and China [38].
Following our previous results in 2018, all sterile males released in 2019 were produced at the CAA (Italy) facility and transported to the release plot [29]. In the current study, for the first time, a successful suppression trial was applied using sterile males from a local Greek strain (Fig. 1) that was mass reared in a facility located in another European country (Italy). Our previous studies indicated the significant challenges associated with transporting sterile males for durations exceeding 24 h. Such extended transportation periods often lead to high mortality rates and increased stress among the sterile males involved, but in our case study, we have significantly reduced the packaging, transportation duration, and release processes to approximately 21 h to minimise the risks of mortality and stress of sterile males. By shortening the transportation time, while safeguarding the quality of the sterile males, we can confirm that we made significant progress towards improving the efficiency and efficacy of our operations. Through planning and implementation, we have successfully established a long-distance transportation method for sterile males, which could also be applied to other European and Mediterranean countries [5].
A progressive increase in efficacy of SIT measured as percentage of induced egg sterility and egg reduction in comparison to control was observed, which lasted for at least 3 weeks after the cessation of the releases. This suggests that the SIT approach can effectively reduce mosquito populations over time to overcome possible compensation effects. During the first 7 weeks of the trial, no reduction in the local Ae. albopictus population was observed. Additional factors, including reduced larval density and potential migration, could influence the observed population dynamics. Nevertheless, since the SIT plot is a relatively isolated area, we assume that this increase was probably related to compensation, a biological mechanism that allows populations to offset losses through increased reproduction and survival. The effect of compensation could have reduced the impact of the first releases, resulting in an apparent lack of population reduction, as observed recently in four other countries [12].
In this study, both induced sterility and egg reduction rates reached a maximum of 78%. In a previous 10-week trial conducted in the same plot (weeks 37–47 of the year 2018, in a smaller release plot of 5 ha), induced sterility exhibited fluctuations within the range of 40–84% without displaying a clear decreasing trend, and it did not result in any significant reduction of egg density [2]. This is in line with previous studies conducted in Italy, which demonstrated that an induced egg sterility below 60% did not lead to a reduction in the egg density [8]. This is also in line with observations from increased larval mortality in Ae. albopictus and is considered to be related to density-dependent compensation of mortality [14, 21, 30]. Specifically, a reduction in egg sterility could lead to a decrease in larval density within breeding sites, which can have varying effects on the overall adult population density.
In the phased conditional approach developed by the International Atomic Energy Agency (IAEA) for SIT suppression trials against Aedes mosquitoes, it is recommended that the number of sterile males released per week exceeds the requirements determined based on the selected field plot and defined release frequency [13]. In light of our results, these numbers should be calculated with the aim of achieving a minimum of 60% sterility.
Wild females immigrating from the surrounding plots is another important factor that could affect the efficacy of SIT field suppression trials, particularly when the size of the study plots is small as it is in the present case [17, 22]. Controlling this parameter is not easy, even though it is crucial for selecting pilot release sites [34]. In the 2018 trials, we targeted only half of the current release plot that is isolated from other villages, with a lower impact on egg hatch and without any reduction of mosquito densities [2]. Extending the release to the full plot clearly decreased the propensity of females immigrating from the surrounding environment and led to much stronger impact on the target population. The ovitraps are the most cost-effective monitoring tools and allow the evaluation of both the density of the wild population and induced sterility [33].
To achieve successful egg hatching outcomes, it is imperative to emphasise the crucial steps of egg collection and oviposition substrate storage. During our recent project in Chania, situated on the island of Crete (Greece), we collected eggs that were subsequently transported and hatched at the Benaki Phytopathological Institute in Athens (Greece) [7]. By prioritising these essential procedures, we were able to optimise our egg hatching process and achieve favourable results. Meticulous attention to detail during egg collection ensures the acquisition of healthy and viable eggs, significantly contributing to the success of subsequent hatching efforts (avoiding issues such as egg desiccation or damage). Furthermore, the proper storage of the oviposition substrate plays a critical role in maintaining the necessary conditions for optimal embryo development.
Moreover, the number of eggs collected can effectively predict the wild population density and therefore provide an estimation of the sterile to wild ratio in the field [6]. Another important aspect to evaluate in an SIT project is the quality of the released males, which determines their mating competitiveness. The evaluation must take into account the lag between releases and their observed effect (at least 3 weeks due to the time needed by the newly emerged females to replace the old already mated females) and, in non-isolated plots, the immigration of wild fertile females from the surrounding plots [13, 17]. In future control efforts in Greece, adult traps will be deployed to assess this parameter, following a procedure that is currently used in the Attica region with great success to monitor the population dynamics of wild Ae. albopictus [2].
In conclusion, the SIT is a method that involves releasing large numbers of sterile males to outcompete wild males in mating with females. Over time, this leads to a decline in pest populations, and in some cases, their complete eradication [18, 36]. The SIT has been widely used to suppress or eradicate populations of major insect pests in agriculture, livestock, and human health, with notable success. It is important to mention that there is no standardised protocol for the release of sterile mosquitoes, and the methodology employed in this study can be considered a prototype. Like any innovative technique, continuous improvements and adjustments are being made to optimise its application, including aspects such as the transportation of sterile males and the assessment of egg hatch rates. In our current study, we released sterile males on a weekly basis for 22 weeks, based on long distance production of sterile males (transported sterile males) resulting in an egg reduction even after the end of the releases.
A limitation of our study was the lack of calculations for the sterile:wild-type male ratio. This limitation arose from our inability to implement Mark Release Recapture (MRR) trials due to limited human resources. Despite this lack of information, we demonstrated that sterile male mosquito releases (SIT implementation) reduced both egg hatching rates and the target mosquito population.
The results obtained from this study provide valuable insights for future SIT programmes targeting Ae. albopictus. Additionally, the adopted methodology can serve as a foundational framework for refining and enhancing release protocols. The consideration of sterile male mosquito transportation is crucial to this improvement. However, the shift from a small-scale trial to a large-scale programme poses new challenges in the associated logistics, such as shipment costs that need to be prioritised and investigated. Our perspective is to establish local production of sterile males, progressively expanding pilot trials, and assessing the cost-effectiveness of integrating SIT into the national integrated vector control strategy.
Acknowledgments
We would like to thank the City Council of the Municipality of Markopoulo Mesogaias (Greece) for their permission to conduct our experiment in the Vravrona area. We gratefully acknowledge Mrs M. Evangeliou for her support of this work. The authors would also like to thank Evangelia Zavitsanou for her contribution in developing Figure 1. This trial was made possible thanks to the support obtained by the EU-funded research infrastructure project Infravec2-Research infrastructures for the control of insect vector-borne diseases (2017–2021) and by the IAEA RER5022 (2061784): Establishing Genetic Control Programs for Aedes Invasive Mosquitoes. Preparatory activities were conducted in the framework of the LIFE CONOPS project “Development & demonstration of management plans against the climate change enhanced invasive mosquitoes in S. Europe” (LIFE12 ENV/GR/000466) co-funded by the EU Environmental Funding Programme LIFE+ Environment Policy and Governance.
Conflict of interest
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Supplementary materials
Video 1: Release of Aedes albopictus sterile males in the field (Credit: George Balatsos). Access here
References
- AIM COST. 2020. A questionnaire-based survey of surveillance and integrated management of Aedes invasive mosquito species. Technical Report. AIM-COST Action CA 17108. University La Sapienza: Rome. Available at https://www.aedescost.eu/sites/default/files/202008/AIMcOSTQuestionnaire_Full_Report_postedV4.pdf. [Google Scholar]
- Balatsos G, Puggioli A, Karras V, Lytra I, Mastronikolos G, Carrieri M, Papachristos DP, Malfacini M, Stefopoulou A, Ioannou C, Balestrino F, Bouyer J, Petrić D, Pajović I, Kapranas A, Papadopoulos NT, Milonas PG, Bellini R, Michaelakis A. 2021. Reduction in egg fertility of Aedes albopictus mosquitoes in Greece following releases of imported sterile males. Insects, 12, 110. [CrossRef] [PubMed] [Google Scholar]
- Balestrino F, Puggioli A, Gilles JR, Bellini R. 2014. Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLoS One, 9(3), e91914. [CrossRef] [PubMed] [Google Scholar]
- Balestrino F, Medici A, Candini G, Carrieri M, Maccagnani B, Calvitti M. 2010. γ Ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. Journal of Medical Entomology, 47, 581–591. [CrossRef] [PubMed] [Google Scholar]
- Becker N, Langentepe-Kong SM, Tokatlian Rodriguez A, Oo TT, Reichle D, Lühken R, Schmidt-Chanasit J, Lüthy P, Puggioli A, Bellini R. 2022. Integrated control of Aedes albopictus in Southwest Germany supported by the Sterile Insect Technique. Parasites & Vectors, 15(1), 1–19. [CrossRef] [PubMed] [Google Scholar]
- Beleri S, Balatsos G, Karras V, Tegos N, Sereti F, Rachiotis G, Hadjichristodoulou C, Papadopoulos N, Papachristos D, Michaelakis A, Patsoula E. 2021. Seasonal phenological patterns and flavivirus vectorial capacity of medically important mosquito species in a wetland and an urban area of Attica, Greece. Tropical Medicine and Infectious Disease, 6(4), 176. [CrossRef] [PubMed] [Google Scholar]
- Bellini R, Carrieri M, Balestrino F, Puggioli A, Malfacini M, Bouyer J. 2021. Field competitiveness of Aedes albopictus (Diptera: Culicidae) irradiated males in pilot sterile insect technique trials in Northern Italy. Journal of Medical Entomology, 58(2), 807–813. [CrossRef] [PubMed] [Google Scholar]
- Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. 2013. Pilot field trials with Aedes Albopictus irradiated sterile males in Italian urban areas. Journal of Medical Entomology, 50, 317–325. [CrossRef] [PubMed] [Google Scholar]
- Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S. 2007. Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial, in Area-Wide Control of Insect Pests: From Research to Field Implementation, Vreysen MJB, Robinson AS, Hendrichs J, Editors. Springer: Dordrecht, The Netherlands. p. 505–515. [CrossRef] [Google Scholar]
- Bellini R, Michaelakis A, Petrić D, Schaffner F, Alten B, Angelini P, Aranda C, Becker N, Carrieri M, Di Luca M, Fălcuţă E. 2020. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Medicine and Infectious Disease, 35, 101691. [CrossRef] [PubMed] [Google Scholar]
- Bond JG, Aguirre-Ibáñez S, Osorio AR, Marina CF, Gómez-Simuta Y, Tamayo-Escobar R, Dor A, Liedo P, Carvalho DO, Williams T. 2021. Sexual competitiveness and induced egg sterility by Aedes aegypti and Aedes albopictus gamma-irradiated males: A laboratory and field study in Mexico. Insects, 12(2), 145. [CrossRef] [PubMed] [Google Scholar]
- Bouyer J. 2023. When less is more: accounting for overcompensation in mosquito SIT projects. Trends in Parasitology, 39(4), 235–237. [CrossRef] [PubMed] [Google Scholar]
- Bouyer J, Vreysen MJB. 2020. Yes, irradiated sterile male mosquitoes can be sexually competitive!. Trends in Parasitology, 36, 877–880. [CrossRef] [PubMed] [Google Scholar]
- Bouyer J, Yamada H, Pereira R, Bourtzis K, Vreysen MJB. 2020. Phased conditional approach for mosquito management using the sterile insect technique. Trends in Parasitology, 36, 325–336. [CrossRef] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference, 2nd edn, New York, NY: Springer. [Google Scholar]
- Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, Morse AP. 2012. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. Journal of the Royal Society Interface, 9(75), 2708–2717. [CrossRef] [PubMed] [Google Scholar]
- Crawford JE, Clarke DW, Criswell V, Desnoyer M, Cornel D, Deegan B, Gong K, Hopkins KC, Howell P, Hyde JS. 2020. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nature Biotechnology, 38, 482–492. [CrossRef] [PubMed] [Google Scholar]
- Dyck VA, Hendrichs J, Robinson AS. 2005. Sterile insect technique: principles and practice in area-wide integrated pest management. Springer: Dordrecht, The Netherlands. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). 2023. Autochthonous transmission of dengue virus in EU/EEA, 2010–present. Available at https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). 2023. Chikungunya virus disease. Available at https://www.ecdc.europa.eu/en/chikungunya-virus-disease. [Google Scholar]
- Evans KG, Neale ZR, Holly B, Canizela CC, Juliano SA. 2023. Survival-larval density relationships in the field and their implications for control of container-dwelling Aedes mosquitoes. Insects, 14(1), 17. [Google Scholar]
- Gato R, Menéndez Z, Prieto E, Argilés R, Rodríguez M, Baldoquín W, Hernández Y, Pérez D, Anaya J, Fuentes I, Lorenzo C, González K, Campo Y, Bouyer J. 2021. Sterile Insect Technique: successful suppression of an Aedes aegypti field population in Cuba. Insects, 12, 469. [CrossRef] [PubMed] [Google Scholar]
- Hurvich CM, Tsai CL. 1995. Model selection for extended quasi-likelihood models in small samples. Biometrics, 51(3), 1077–1084. [Google Scholar]
- Južnič-Zonta Ž, Sanpera-Calbet I, Eritja R, Palmer JR, Escobar A, Garriga J, Oltra A, Richter-Boix A, Schaffner F, della Torre A, Miranda MÁ. 2022. Mosquito alert: leveraging citizen science to create a GBIF mosquito occurrence dataset. Gigabyte, 2022, 1–11. [Google Scholar]
- Knipling EF. 1979. The basic principles of insect population suppression and management. Washington, DC: US Government Printing Office. [Google Scholar]
- Kolimenakis A, Heinz S, Wilson ML, Winkler V, Yakob L, Michaelakis A, Papachristos D, Richardson C, Horstick O. 2021. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit – a systematic review. PLoS Neglected Tropical Diseases, 15(9), e0009631. [CrossRef] [PubMed] [Google Scholar]
- Maiga H, Mamai W, Yamada H, Argiles Herrero R, Bouyer J. 2020. Guidelines for mass-rearing of Aedes mosquitoes. Version 1.0. Vienna, Austria: Insect Pest Control Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. [Google Scholar]
- Malfacini M, Puggioli A, Balestrino F, Carrieri M, Dindo ML, Bellini R. 2022. Aedes albopictus sterile male production: influence of strains, larval diet and mechanical sexing tools. Insects, 13(10), 899. [CrossRef] [PubMed] [Google Scholar]
- Mastronikolos GD, Kapranas A, Balatsos GK, Ioannou C, Papachristos DP, Milonas PG, Puggioli A, Pajović I, Petrić D, Bellini R, Michaelakis A, Papadopoulos NT. 2022. Quality control methods for Aedes albopictus sterile male transportation. Insects, 13, 179. [CrossRef] [PubMed] [Google Scholar]
- Neale ZR, Juliano SA. 2019. Finding the sweet spot: What levels of larval mortality lead to compensation or overcompensation in adult production? Ecosphere, 10(9), e02855. [CrossRef] [PubMed] [Google Scholar]
- Oliva CF, Benedict MQ, Collins CM, Baldet T, Bellini R, Bossin H, Bouyer J, Corbel V, Facchinelli L, Fouque F, Geier M. 2021. Sterile Insect Technique (SIT) against Aedes species mosquitoes: A roadmap and good practice framework for designing, implementing and evaluating pilot field trials. Insects, 12(3), 191. [CrossRef] [PubMed] [Google Scholar]
- Pichler V, Caputo B, Valadas V, Micocci M, Horvath C, Virgillito C, Akiner M, Balatsos G, Bender C, Besnard G, Bravo-Barriga D. 2022. Geographic distribution of the V1016G knockdown resistance mutation in Aedes albopictus: a warning bell for Europe. Parasites & Vectors, 15(1), 280. [CrossRef] [PubMed] [Google Scholar]
- Stefopoulou A, Balatsos G, Papadopoulos NT, Daskalakis D, Daskalakis D, Chatzidaki A, Milonas P, Papachristos D, Michaelakis A. 2022. Spatial and temporal dynamics of Aedes albopictus populations in rural and agricultural areas in Chania, Greece, after its invasion. Frontiers in Tropical Diseases, 3, 52. [CrossRef] [Google Scholar]
- Stefopoulou A, LaDeau SL, Syrigou N, Balatsos G, Karras V, Lytra I, Boukouvala E, Papachristos DP, Milonas PG, Kapranas A, Vahamidis P. 2021. Knowledge, attitude, and practices survey in Greece before the Implementation of Sterile Insect Technique against Aedes albopictus. Insects, 12(3), 212. [CrossRef] [PubMed] [Google Scholar]
- Tur C, Almenar D, Benlloch-Navarro S, Argilés-Herrero R, Zacarés M, Dalmau V, Pla I. 2021. Sterile insect technique in an integrated vector management program against tiger mosquito Aedes albopictus in the Valencia region (Spain): operating procedures and quality control parameters. Insects, 12(3), 272. [CrossRef] [PubMed] [Google Scholar]
- Vreysen MJB, Klassen W. 2021. Area-wide integrated pest management and the Sterile Insect Technique, in Sterile insect technique: principles and practice in area-wide integrated pest management. Dyck VA, Hendrichs J, Robinson AS, Editors. CRC Press: Boca Raton, FL. p. 75–112. [Google Scholar]
- WHO, IAEA. 2020. Guidance framework for testing the sterile insect technique as a vector control tool against Aedes-borne diseases, Geneva & Vienna. [Google Scholar]
- Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, Liang Y, Pan X, Hu L, Sun Q, Wang X, Wei Y, Zhu J, Qian W, Yan Z, Parker AG, Gilles JRL, Bourtzis K, Bouyer J, Tang M, Zheng B, Yu J, Liu J, Zhuang J, Hu Z, Zhang M, Gong JT, Hong XY, Zhang Z, Lin L, Liu Q, Hu Z, Wu Z, Baton LA, Hoffmann AA, Xi Z. 2019. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature, 572, 56–61. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Balatsos G, Karras V, Puggioli A, Balestrino F, Bellini R, Papachristos DP, Milonas PG, Papadopoulos NT, Malfacini M, Carrieri M, Kapranas A, Mamai W, Mastronikolos G, Lytra I, Bouyer J & Michaelakis A. 2024. Sterile Insect Technique (SIT) field trial targeting the suppression of Aedes albopictus in Greece. Parasite 31, 17.
All Tables
Description of the study plot and the respective control plots including the number of ovitraps deployed to assess the efficacy of the SIT trial.
Descriptive data of the 22 weekly releases of sterile males carried out in Greece in 2019.
Fixed-effects coefficients of a mixed-effect binomial model of the impact of treatment, time and their interaction on the hatch rate of eggs of Aedes albopictus (1,426 observations, 60 traps, 24 collection dates, Control 2 site used as a reference).
Fixed-effects coefficients of a mixed-effect Poisson model of the impact of treatment, time and their interaction on the density of eggs of Aedes albopictus (1,426 observations, 60 traps, 24 collection dates, Control 2 site used as a reference).
All Figures
 |
Figure 1 Geographic position of the SIT plots and the respective control plots C1 and C2 (A) and distribution of releasing points within the SIT plot (B). |
| In the text | |
 |
Figure 2 Temperature and Relative Humidity (RH) data at the SIT and control plots [SIT: 65.030 ± 0.185 RH and 23.414 ± 0.062 °C], [Control 1 (C1): 63.796 ± 0.178 RH and 23.315 ± 0.056 °C], [Control 2 (C2): 57.887 ± 0.205 RH and 25.175 ± 0.067 °C]. |
| In the text | |
 |
Figure 3 Hatching rates at SIT and control plots. The hatching rates of collected eggs (oviposition substrates) from 60 ovitraps were assessed weekly. |
| In the text | |
 |
Figure 4 Seasonal patterns of induced sterility and egg reduction at the SIT plot as compared with the control area (right Y axis). The grey bars indicate the number of sterile males released per hectare (ha) per week from 03 May to 04 October (2019) at the SIT plot (left Y axis). |
| In the text | |
 |
Figure 5 The seasonal trend of egg density (mean ± SE) collected at SIT and control plots C1 and C2. |
| In the text | |
 |
Figure 6 Weekly distribution of oviposition positivity index (OPI) at the release (SIT) and control plots C1 and C2 from 03 May to 29 November (2019). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.