| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 9 | |
| Number of page(s) | 13 | |
| DOI | https://doi.org/10.1051/parasite/2024007 | |
| Published online | 09 February 2024 | |
Research Article
Global prevalence and risk factors of Enterocytozoon bieneusi infection in humans: a systematic review and meta-analysis
Prévalence mondiale et facteurs de risque de l’infection à Enterocytozoon bieneusi chez l’homme : revue systématique et méta-analyse
1
School of Pharmacy, Yancheng Teachers University, Yancheng 224002, Jiangsu Province, PR China
2
College of Life Sciences, Changchun Sci-Tech University, Shuangyang 130600, Jilin Province, PR China
3
Department of Technology, Ningbo Sansheng Biotechnology Co., Ltd, Ningbo 315000, Zhejiang Province, PR China
4
College of Veterinary Medicine, Qingdao Agricultural University, Qingdao 266109, Shandong Province, PR China
5
Department of Medical Microbiology and Immunology, School of Basic Medicine, Dali University, Dali 671000, Yunnan Province, PR China
6
College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, Jilin Province, PR China
* Corresponding author: jiangjingxiaoyao@163.com; caohw@yctu.edu.cn
Received:
27
April
2023
Accepted:
22
January
2024
Enterocytozoon bieneusi is one of the most important zoonotic pathogens. In this study, we present a systematic review and meta-analysis of the prevalence of human E. bieneusi infection in endemic regions and analyze the various potential risk factors. A total of 75 studies were included. Among 31,644 individuals tested, 2,291 (6.59%) were E. bieneusi-positive. The highest prevalence of E. bieneusi in the male population was 5.50%. The prevalence of E. bieneusi in different age groups was varied, with 10.97% in teenagers. The prevalence of E. bieneusi in asymptomatic patients (6.49%) is significantly lower than that in HIV-infected patients (11.49%), and in patients with diarrheal symptoms (16.45%). Rural areas had a higher rate (7.58%) than urban ones. The prevalence of E. bieneusi in humans was the highest (6.42%) at altitudes <10 m. Moreover, the temperate zone marine climate (13.55%) had the highest prevalence. A total of 69 genotypes of E. bieneusi have been found in humans. This is the first global study regarding E. bieneusi prevalence in humans. Not only people with low immunity (such as the elderly, children, people with HIV, etc.), but also people in Europe in temperate marine climates should exercise caution to prevent infection with E. bieneusi during contact process with animals.
Résumé
Enterocytozoon bieneusi est l’un des agents pathogènes zoonotiques les plus importants. Dans cette étude, nous présentons une revue systématique et une méta-analyse de la prévalence de l’infection humaine à E. bieneusi dans les régions endémiques et analysons les différents facteurs de risque potentiels. Au total, 75 études ont été incluses. Parmi 31 644 individus, 2 291 (6,59 %) étaient positifs à E. bieneusi. La prévalence la plus élevée d’E. bieneusi dans la population masculine était de 5,50 %. La prévalence d’E. bieneusi dans différents groupes d’âge variait, avec 10,97 % chez les adolescents. La prévalence d’E. bieneusi chez les patients asymptomatiques (6,49 %) était significativement inférieure à celle des patients VIH (11,49 %) et des patients présentant des symptômes de diarrhée (16,45 %). Les zones rurales avaient un taux plus élevé (7,58 %) que les zones urbaines. La prévalence d’E. bieneusi chez les humains était la plus élevée (6,42 %) à une altitude <10 m. De plus, le climat marin de la zone tempérée (13,55 %) avait la prévalence la plus élevée. Au total, 69 génotypes d’E. bieneusi ont été trouvés chez l’homme. Il s’agit de la première étude mondiale concernant la prévalence d’E. bieneusi chez l’homme. Non seulement les personnes ayant une faible immunité (telles que les personnes âgées, les enfants, les patients atteints du VIH, etc.), mais également les personnes vivant en Europe dans un climat marin tempéré doivent veiller à prévenir l’infection par E. bieneusi lors du contact avec des animaux.
Key words: Enterocytozoon bieneusi / Prevalence / Risk factors / Humans / Meta-analysis
© Y. Wang et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The Microsporidia, infecting a broad range of both vertebrate and invertebrate hosts, live exclusively within host cells [30]. This group of parasites currently comprises more than 200 genera and 1,600 species, including relatively common species, such as Encephalitozoon cuniculi, Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Encephalitozoon hellem [5, 75]. Enterocytozoon bieneusi is the most important zoonotic microsporidian species worldwide and is transmitted predominantly through the fecal-oral route because it is capable of infecting a broad range of hosts, including humans, domestic animals, poultry, companion animals, birds, and wildlife [14, 63].
Enterocytozoon bieneusi was first identified in human immunodeficiency virus (HIV) patients in 1985, and with the expansion of the HIV epidemic, the number of cases of E. bieneusi infections in humans has increased [29]. To date, more than 200 genotypes have been identified and classified into 11 groups (groups 1–11) [40]. The genotypes identified in Group 1 are predominantly present in the human population, with the most frequently occurring ones being A, D, EbpC, and Type IV [40]. Although genotypes from other groups can also infect humans, they are relatively rare and are generally considered to have little public health significance [42].
Enterocytozoon bieneusi infection is associated with persistent diarrhea, malabsorption, and wasting diathesis in individuals with compromised immune systems, particularly those diagnosed with acquired immunodeficiency syndrome (AIDS) and can lead to life-threatening chronic diarrhea [6, 51, 87]. Patients with normal immune function can also develop self-limited diarrhea lasting up to one month [70]. Unfortunately, there are no currently effective treatments available for E. bieneusi infection [41]. Due to its importance and potential threat to public health, E. bieneusi has been classified as a Category B agent by the National Institutes of Health (NIH, https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogen), making it a second-highest priority organism/biological agent [97]. In addition, many public health organizations and academic institutions in various countries and regions have included E. bieneusi in their monitoring and control plans for infectious pathogens to ensure public health and safety.
Here, we present a systematic review and meta-analysis that evaluates the prevalence of human E. bieneusi infection in endemic regions. Our analysis considered various potential risk factors, such as gender, age, season, and geographic location.
Material and methods
Search strategy
We searched for all studies on the prevalence of E. bieneusi infection in humans around the world up to July 2022 from six databases, i.e., China National Knowledge Infrastructure (CNKI), VIP Chinese Journal Database (VIP), Wanfang Data, PubMed, Web of Science, and ScienceDirect. The retrieval strategy for the three Chinese databases was to use the search keywords “humans (in Chinese)” and “Microsporidia (in Chinese)”, in advanced retrieval. The three English-language databases were searched using search formulas, as follows: Web of Science: (TI=(Humans) OR TI=(Man) OR TI=(Homo sapiens)) AND (TI=(Microsporidia) OR TI=(Microsporidium) OR TI=(Microsporidiums)); ScienceDirect: (Human OR “Homo sapiens” OR Man) AND (Microsporidia OR Microsporidiums OR Microsporidium); PubMed: ((“Humans”[Mesh]) OR (Homo sapiens[Title/Abstract]) OR (Man[Title/Abstract]) OR (Man, Modern[Title/Abstract]) OR (Modern Man[Title/Abstract]) OR (Human[Title/Abstract])) AND (“Microsporidia”[Mesh]) OR (Microsporidias[Title/Abstract]) OR (Microspora[Title/Abstract]) OR (Microsporidians[Title/Abstract]) OR (Microsporidian[Title/Abstract]) AND (“epidemiology” [MeSH]) OR (epidemics[Title/Abstract])) OR (prevalence[Title/Abstract])) OR (frequency[Title/Abstract])) OR (surveillance[Title/Abstract])) OR (incidence[Title/Abstract])) OR (occurrence[Title/Abstract])).
Inclusion and exclusion criteria
The literature aggregation software used was Endnote X9.3.2 [15]. The inclusion criteria for this meta-analysis were as follows: (1) study on the prevalence of E. bieneusi in humans; (2) data must include a clear total number of surveyed humans and the number of positives; (3) the articles must have full text; and (4) the research must be designed to scale out. The exclusion criteria for this meta-analysis were as follows: (1) not Chinese or English literature; (2) data error, no data or data duplication in studies; (3) the research subject is not E. bieneusi and humans; (4) conference report or summary; and (5) no detailed positivity rate.
Data extraction
Two authors independently extracted and recorded the data. The lead author of this meta-analysis further evaluated any differences or uncertainties regarding research qualifications. The extracted data included the article title, testing method, residence, HIV or diarrhea, article quality, age and gender of the patient, country, longitude and latitude, altitude and climate, and the total and positive numbers. The geographic data (longitude, latitude, and altitude) collected were from the National Oceanic and Atmospheric Administration (NOAA; https://www.ncei.noaa.gov/maps/monthly/). We also considered various socioeconomic variables (World Bank–income category, National population in 2021, and the human development index (HDI): https://data.worldbank.org/, https://population.un.org/, and https://hdr.undp.org/).
Statistical analyses
This meta-analysis was performed according to the PRISMA statement [67]. The “meta” package (version 6.0-0) in RStudio (version 4.0.5) was employed to analyze the data. Before performing the meta-analysis, we tested five transformation methods (Table S1). The W-value close to 1 and the p-value > 0.05 were close to the normal distribution criterion. We used Cochran-Q, I2 statistics, and χ2 tests to calculate the heterogeneity between studies. When p-value < 0.05 and I2 > 50%, this indicates the existence of heterogeneity, and the random effect model was adopted; when p-value > 0.05, I2 < 50%, this indicates that there is no heterogeneity, and the fixed effect model was adopted. A forest plot was used to visualize the statistical results of the meta-analysis; a funnel plot and Egger test detected the publication bias of the research; the sensitivity analysis evaluated the stability of the meta-analysis model and the reliability of the results; and subgroup analysis and univariate regression analysis verified the potential source of heterogeneity.
Results
Search results
A total of 1,485 publications were identified, and after the titles and abstracts were reviewed, 1,326 papers were selected for full-text reading. According to the inclusion and exclusion criteria, 75 studies were finally included after screening (Fig. 1 and Table S2). Among them, 40 publications had 4 points, 26 publications had 3 points, and 9 publications had 2 points (Table S2).
 |
Figure 1 Flow diagram of literature search and selection. |
Qualification research and publication bias
This study used PLOGIT for data transformation (Table S1; W = 0.98608, p = 0.5848). The forest plot of global human infection with E. bieneusi showed high heterogeneity (χ2 = 1.6138, I2 = 96.0%, p < 0.01), so a random effects model was used (Fig. 2). According to the funnel plot and Egger test, there was publication bias in this study (p = 0.0001; Table S3, Figs. S1, and S2). There were 24 supplementary articles shown in the trim and fill analysis (Fig. S3). Based on the sensitivity test, the data after restructuring were not significantly affected (Fig. S4). Figure 3 shows the distribution of human E. bieneusi prevalence. Distribution is essentially worldwide, but there are few reports from North America.
 |
Figure 2 Forest map of global human infection in E. bieneusi. |
 |
Figure 3 Map of E. bieneusi prevalence in human worldwide. |
Meta-analysis of E. bieneusi prevalence in humans worldwide
Detailed data on global human E. bieneusi prevalence are summarized in Tables 1 and 2. The global prevalence of E. bieneusi infection in humans was 6.59% (95% CI: 4.97–8.68). The highest prevalence of E. bieneusi in the male population was 5.50% (95% CI: 3.54–8.45). Concerning the environment of residence, rural areas had the highest rate at 7.58% (95% CI: 4.22–13.25). The prevalence of E. bieneusi in teenagers was 10.97% (95% CI: 5.90–17.36). The infection rate was 16.5% (95% CI: 11.47–22.18) in patients with diarrhea, and 10.54% (95% CI: 5.79–16.44) in patients without diarrhea. The rate of infection in patients with cancer was 69.89% (95% CI: 60.13–78.84), and the rate of infection in other patients was also high, i.e., 39.00% (95% CI: 32.34–45.87) in bone marrow transplant patients, 14.4% (95% CI: 9.84–19.62) in patients with HIV, etc. Concerning the analyzed geographic factors, the positive rate of E. bieneusi was the highest in humans living at altitudes <10 m (6.42%; 95% CI: 4.02–10.08). According to the analysis of climatic factors, we found that the temperate zone marine climate had the highest positive rate of human E. bieneusi (13.55%; 95% CI: 3.19–42.73; Table 1).
Summary of global E. bieneusi human infection rates and relevant characteristics.
Prevalence estimates of E. bieneusi infection, and estimated numbers of infected people in 34 countries.
According to income level, the highest prevalence rates of E. bieneusi infection was in countries with high (9.53%; 95% CI: 5.01–17.37) and lower-middle (7.31%; 95% CI: 5.17–10.24) income levels, with the lowest prevalence estimated for upper-middle income countries (5.07%; 95% CI: 3.24–7.85). According to HDI, the level subgroup analysis indicated that the highest prevalence rates were estimated for countries with extremely high HDI levels (7.93%; 95% CI: 4.94–12.48%) (Table 2). Countries with high prevalence rates included Germany (41.66%), South Africa (32.94%), Czechia (31.08%), and Russia (18.87%). The highest positive rate of human E. bieneusi infection was 14.70% (95% CI: 8.21–22.68) in Europe, which indicates that 109,540,000 (range: 61,178,000–169,005,000) people will be infected in Europe in 2021. An extrapolation to the 2021 world population indicated that 521,222,000 (range: 393,092,000–686,526,000) people harbored E. bieneusi infection. More detail on the global and regional E. bieneusi infection prevalence is given in Table 2.
A total of 70 E. bieneusi genotypes were included in this study, of which Group 1 was the most common in human infections (n = 40, 57.14%), followed by Group 2 (n = 5, 7.14%) (Table 3 and Fig. 4). According to the genotypes of E. bieneusi in different countries, China had the most E. bieneusi genotypes (n = 52; 48%). China shares three genotypes with Brazil and Thailand, four with Bangladesh, and one with Myanmar (Fig. 5).
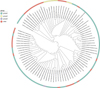 |
Figure 4 Evolutionary tree of E. bieneusi prevalence in human worldwide. |
 |
Figure 5 Prevalence of E. bieneusi genotype in different countries. |
Prevalence of E. bieneusi genotype grouping.
Discussion
Microsporidia specializing in intracellular parasitism [5] are a type of single-celled eukaryotic organism [29, 76]. As we know, more than 90% of human microsporidiosis cases are caused by E. bieneusi as the most important species of microsporidiosis, with worldwide distribution of infected cases [37, 65, 83, 94]. We have estimated that of the world population of 7.9 billion people, over 520 million people may be infected with E. bieneusi in 2021 [10].
In this study, the results showed that the infection rate in men was higher than that in women, but the difference was not statistically significant. A similar phenomenon was also found in the meta-analysis of the prevalence of microsporidia in China by Qiu et al. [65]. For people, the high male infection rate may be related to poor living habits and higher engagement in animal husbandry. At the same time, the high positive rate of the population in rural areas may also be related to high engagement in animal husbandry [55]. In rural areas, it is customary to raise poultry, livestock and pets, and most of them are raised in a way that combines free range breeding with captive breeding. Close contact between people and animals, poor living conditions, pollution of drinking water by animal feces, and low awareness of prevention methods all increase the probability of E. bieneusi infection [41].
There are currently more than 500 genotypes of E. bieneusi identified based on ITS nucleic acid sequences, and these existing genotypes can be divided into different genetic groups, totaling 11 groups (Groups 1–11). The results of this study showed that 82.05% of genotypes belonged to Group 1. Group 1 has the largest number of species and is found in both domestic and wild animals worldwide [42], and is also the genotype group of E. bieneusi that mainly infects humans [31, 32]. However, genotypes in other groups causing zoonotic disease cannot be ignored, such as J and BEBE4 in group 2, CAF4 and KIM3 in group 5, Nig3 and Nig4 in group 6, and S7 in group 10 [31, 40, 53, 63]. These genotypes with the potential for zoonotic co-infection, can infect both animal hosts and humans, establishing a pathway of transmission between humans and animals [41, 104, 105]. This is therefore of high public health significance. Findings also further demonstrate that human E. bieneusi infection is mainly related to animals [31, 32].
Enterocytozoon bieneusi is one of the common pathogens that causes chronic diarrhea in the human body, especially in people with immunodeficiency or low immunity (such as people with HIV, organ transplant recipients, bone marrow transplant recipients, patients with tumors, elderly people, and children) [83, 94, 100]. In the statistical analysis of people at different ages, compared with other groups, the infection rates of young people and the elderly were higher. Previous studies have shown that young and older age are risk factors for E. bieneusi infection [29]. The prevalence of E. bieneusi in diarrhea patients 16.50% (11.47–22.18) was significantly higher than that in non-diarrhea patients 10.54% (5.79–16.44). Several studies have shown similar results, indicating that patients with diarrhea have significantly higher rates of E. bieneusi infection than those without diarrhea. People with diarrhea are more susceptible to infection [99, 102]. Meanwhile, E. bieneusi infection is widespread and infectious for people with HIV, as well as for others with compromised immunity [29]. This study found that E. bieneusi had the highest infection rate among patients with cancer, followed by organ transplant recipients. However, the mechanism of infection and pathogenicity established by the entry of E. bieneusi into host cells is not yet clear, and there may be a certain balance between it and the host, causing the host to be in a subclinical state for a long period of time [74]. Once the host’s immune function is compromised, obvious clinical symptoms will appear, such as persons with ≤200 CD4+ T cells per microliter blood [16, 26]. Moreover, the infection rate of E. bieneusi in persons with <200 (25.97%) CD4+ T cells was higher than that in those with >200 CD4+ T cells (21.76%). Therefore, it is recommended that patients with low immunity should maintain good hygiene habits and seek medical attention in good time when symptoms such as diarrhea occur [101, 106].
Our analysis also showed that based on economic development, high infection rates are found in the extremely high HDI and high-income countries. On the one hand, this may be due to differences in testing technology, focus, and logistics development [69]. On the other, it may be due to climate. Our research showed that the prevalence rate of E. bieneusi in European countries was relatively high. The results of the climate subgroups showed that the prevalence in temperate zone marine climate was the highest. Studies have shown that extreme temperatures and precipitation are not conducive to the growth of E. bieneusi [43, 63]. The temperate zone marine climate has the characteristics of warm winters and cool summers, small annual temperature differences, and uniform precipitation distribution. This may be beneficial for the growth and transmission of E. bieneusi.
Although we conducted a comprehensive and detailed analysis of the risk factors for E. bieneusi infection in humans in this study, there were certain limitations. First, we may have missed some studies. Second, in some cases, our estimates might not be representative of national prevalence nor of all communities in a country. Third, some included studies lack infection rates based on gender, age, and living environment factors, and information on the different detection methods used, which may affect certain subgroup analyses.
Conclusions
This study revealed a global prevalence rate of 6.59% for human E. bieneusi infection, with 82.05% of the genotypes belonging to Group 1 and posing a risk of zoonotic disease. Not only people with low immunity (such as the elderly, children, patients with HIV, etc.), but also people in Europe living in temperate marine climates should exercise caution to prevent infection with E. bieneusi during contact with animals.
Supplementary material
Table S1: Normal distribution tests for normal rates and different transitions of articles hosted by humans. Access here
Table S2: Main characteristics of the included studies in humans. Access here
Table S3: Egger for publication bias. Access here
 |
Figure S1: Funnel plot with pseudo 95% confidence interval for publication bias test. |
 |
Figure S2: Egger’s test for publication bias. |
 |
Figure S3: Shear complement graph and pseudo 95% confidence interval for publication bias test. |
 |
Figure S4: Figure S4: Sensitivity analysis of human infection with E. bieneusi. |
Funding
This work was supported by the Jilin Provincial Education Department of Science and Technology Research Project (JJKH20210366KJ).
Conflict of interest
The authors declare that they have no conflicts of interest.
Data availability statement
The data used to support the findings of this study are available from the corresponding authors upon request.
Author contributions statement
Yanchun Wang, Hongwei Cao, and Jing Jiang designed the study. Yanan Cai acquired the funding. Xiao-Man Li, Xiang-Yu Wang, Yong-Jie Wei, and Hai-Long Yu extracted the data. Xiao-Man Li, Xiang-Yu Wang, Xing Yang, and Xin-Bo Yang analyzed the data. Yanchun Wang, Xiao-Man Li, and Xing Yang wrote the original draft. Xiang-Yu Wang, Yong-Jie Wei, Yanan Cai, Hong-Li Geng, and Jing Jiang reviewed the draft. All authors reviewed the final manuscript.
Ethics approval
Ethical approval was not required.
References
- Abreu-Acosta N, Lorenzo-Morales J, Leal-Guio Y, Coronado-Alvarez N, Foronda P, Alcoba-Florez J, Izquierdo F, Batista-Diaz N, Del AC, Valladares B. 2005. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Transactions of the Royal Society of Tropical Medicine and Hygiene, 99, 848–855. [CrossRef] [PubMed] [Google Scholar]
- Al-Brhami K, Abdul-Ghani R, Al-Qobati SA. 2022. Intestinal microsporidiosis among HIV/AIDS patients receiving antiretroviral therapy in Sana’a city, Yemen: first report on prevalence and predictors. BMC Infectious Diseases, 22, 11. [CrossRef] [PubMed] [Google Scholar]
- Alfa CO, Ouattara A, Thellier M, Accoceberry I, Biligui S, Minta D, Doumbo O, Desportes-Livage I, Thera MA, Danis M. 2002. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali). Journal of Clinical Microbiology, 40, 1715–1718. [CrossRef] [PubMed] [Google Scholar]
- Ayinmode AB, Zhang H, Dada-Adegbola HO, Xiao L. 2014. Cryptosporidium hominis subtypes and Enterocytozoon bieneusi genotypes in HIV-infected persons in Ibadan, Nigeria. Zoonoses and Public Health, 61, 297–303. [CrossRef] [PubMed] [Google Scholar]
- Bojko J, Reinke AW, Stentiford GD, Williams B, Rogers M, Bass D. 2022. Microsporidia: a new taxonomic, evolutionary, and ecological synthesis. Trends in Parasitology, 38, 642–659. [CrossRef] [PubMed] [Google Scholar]
- Brasil P, de Lima DB, de Paiva DD, Lobo MS, Sodré FC, Silva SP, Villela EV, Silva EJ, Peralta JM, Morgado M. 2000. Clinical and diagnostic aspects of intestinal microsporidiosis in HIV-infected patients with chronic diarrhea in Rio de Janeiro, Brazil. Revista do Instituto de Medicina Tropical de São Paulo, 42, 299–304. [CrossRef] [PubMed] [Google Scholar]
- Breton J, Bart-Delabesse E, Biligui S, Carbone A, Seiller X, Okome-Nkoumou M, Nzamba C, Kombila M, Accoceberry I, Thellier M. 2007. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. Journal of Clinical Microbiology, 45, 2580–2589. [CrossRef] [PubMed] [Google Scholar]
- Cetinkaya U, Hamamci B, Kaynar L, Kuk S, Sahin I, Yazar S. 2015. Investigation of the presence of Encephalitozoon intestinalis and Enterocytozoon bieneusi in bone marrow transplant patients by IFA-MAbs method. Mikrobiyoloji Bulteni, 49, 432–438. (in Russian). [CrossRef] [PubMed] [Google Scholar]
- Clarridge JR, Karkhanis S, Rabeneck L, Marino B, Foote LW. 1996. Quantitative light microscopic detection of Enterocytozoon bieneusi in stool specimens: a longitudinal study of human immunodeficiency virus-infected microsporidiosis patients. Journal of Clinical Microbiology, 34, 520–523. [CrossRef] [PubMed] [Google Scholar]
- Conteas CN, Berlin OG, Lariviere MJ, Pandhumas SS, Speck CE, Porschen R, Nakaya T. 1998. Examination of the prevalence and seasonal variation of intestinal microsporidiosis in the stools of persons with chronic diarrhea and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene, 58, 559–561. [CrossRef] [PubMed] [Google Scholar]
- Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens MA, Trepo C. 1999. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. Journal of Infectious Diseases, 180, 2003–2008. [CrossRef] [PubMed] [Google Scholar]
- Coyle CM, Wittner M, Kotler DP, Noyer C, Orenstein JM, Tanowitz HB, Weiss LM. 1996. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clinical Infectious Diseases, 23(5), 1002–1006. [CrossRef] [PubMed] [Google Scholar]
- Del AC, Navajas R, Gurbindo D, Ramos JT, Mellado MJ, Fenoy S, Munoz FM, Subirats M, Ruiz J, Pieniazek NJ. 1997. Microsporidiosis in HIV-positive children in Madrid (Spain). Journal of Eukaryotic Microbiology, 44, 84S–85S. [Google Scholar]
- Desportes I, Le Charpentier Y, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. 1985. Needs renumbering and adding all authors Occurrence of a new microsporidan: Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. Journal of Protozoology, 32, 250–254. [CrossRef] [PubMed] [Google Scholar]
- Diao N-C, Zhao B, Chen Y, Wang Q, Chen Z-Y, Yang Y, Sun Y-H, Shi J-F, Li J-M, Shi K, Gong Q-L, Du R. 2022. Prevalence of Eimeria spp. among goats in China: a systematic review and meta-analysis. Frontiers in Cellular and Infection Microbiology, 12, 806085. [CrossRef] [PubMed] [Google Scholar]
- Didier ES. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Tropica, 94, 61–76. [CrossRef] [PubMed] [Google Scholar]
- Ding S, Huang W, Qin Q, Tang J, Liu H. 2018. Genotype identification and phylogenetic analysis of Enterocytozoon bieneusi isolates from stool samples of diarrheic children. Journal of Parasitology, 104, 297–301. [CrossRef] [PubMed] [Google Scholar]
- Espern A, Morio F, Miegeville M, Illa H, Abdoulaye M, Meyssonnier V, Adehossi E, Lejeune A, Cam PD, Besse B, et al. 2007. Molecular study of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon intestinalis among human immunodeficiency virus-infected patients from two geographical areas: Niamey, Niger, and Hanoi, Vietnam. Journal of Clinical Microbiology, 45, 2999–3002. [CrossRef] [PubMed] [Google Scholar]
- Ferreira FM, Bezerra L, Santos MB, Bernardes RM, Avelino I, Silva ML. 2001. Intestinal microsporidiosis: a current infection in HIV-seropositive patients in Portugal. Microbes and Infection, 3, 1015–1019. [CrossRef] [PubMed] [Google Scholar]
- Field AS, Marriott DJ, Hing MC. 1993. The Warthin-Starry stain in the diagnosis of small intestinal microsporidiosis in HIV-infected patients. Folia Parasitologica, 40, 261–266. [PubMed] [Google Scholar]
- Franzen C, Muller A, Hegener P, Hartmann P, Salzberger B, Franzen B, Diehl V, Fatkenheuer G. 1996. Polymerase chain reaction for microsporidian DNA in gastrointestinal biopsy specimens of HIV-infected patients. AIDS, 10, F23–F27. [CrossRef] [PubMed] [Google Scholar]
- Franzen C, Muller A, Hegener P, Salzberger B, Hartmann P, Fatkenheuer G, Diehl V, Schrappe M. 1995. Detection of microsporidia (Enterocytozoon bieneusi) in intestinal biopsy specimens from human immunodeficiency virus-infected patients by PCR. Journal of Clinical Microbiology, 33, 2294–2296. [CrossRef] [PubMed] [Google Scholar]
- Ghoshal U, Kalra SK, Tejan N, Ranjan P, Dey A, Nityanand S. 2021. Prevalence and Genetic Characterization of Cryptosporidium and Microsporidia infecting hematological malignancy patients. Acta Parasitologica, 66, 508–516. [CrossRef] [PubMed] [Google Scholar]
- Ghoshal U, Khanduja S, Pant P, Prasad KN, Dhole TN, Sharma RK, Ghoshal UC. 2015. Intestinal microsporidiosis in renal transplant recipients: prevalence, predictors of occurrence and genetic characterization. Indian Journal of Medical Microbiology, 33, 357–363. [CrossRef] [PubMed] [Google Scholar]
- Ghoyounchi R, Mahami-Oskouei M, Rezamand A, Spotin A, Aminisani N, Nami S, Pirestani M, Berahmat R, Madadi S. 2019. Molecular phylodiagnosis of Enterocytozoon bieneusi and Encephalitozoon intestinalis in children with cancer: microsporidia in malignancies as an emerging opportunistic infection. Acta Parasitologica, 64, 103–111. [CrossRef] [PubMed] [Google Scholar]
- Halanova M, Valencakova A, Jarcuska P, Halan M, Danisova O, Babinska I, Dedinska K, Cislakova L. 2019. Screening of opportunistic Encephalitozoon intestinalis and Enterocytozoon bieneusi in immunocompromised patients in Slovakia. Central European Journal of Public Health, 27, 330–334. [CrossRef] [PubMed] [Google Scholar]
- Halanova M, Valencakova A, Malcekova B, Kvac M, Sak B, Kvetonova D, Balent P, Cislakova L. 2013. Occurrence of microsporidia as emerging pathogens in Slovak Roma children and their impact on public health. Annals of Agricultural and Environmental Medicine, 20, 695–698. [PubMed] [Google Scholar]
- Hamamci B, Cetinkaya U, Berk V, Kaynar L, Kuk S, Yazar S. 2015. Prevalence of Encephalitozoon intestinalis and Enterocytozoon bieneusi in cancer patients under chemotherapy. Mikrobiyoloji Bulteni, 49, 105–113. [CrossRef] [PubMed] [Google Scholar]
- Han B, Pan G, Weiss LM. 2021. Microsporidiosis in humans. Clinical Microbiology Reviews, 34, e0001020. [CrossRef] [PubMed] [Google Scholar]
- Han B, Weiss LM. 2017. Microsporidia: obligate intracellular pathogens within the Fungal Kingdom. Microbiology Spectrum, 5(2). [PubMed] [Google Scholar]
- Haro M, Izquierdo F, Henriques-Gil N, Andrés I, Alonso F, Fenoy S, del Aguila C. 2005. First detection and genotyping of human-associated microsporidia in pigeons from urban parks. Applied and Environmental Microbiology, 71, 3153–3157. [CrossRef] [PubMed] [Google Scholar]
- Hwang S, Shin S, Kim S, Ryu J, Choi K. 2020. Identification of zoonotic Enterocytozoon bieneusi genotypes in pre-weaned Korean native calves. Research Square. https://doi.org/10.21203/rs.2.22405/v1. [Google Scholar]
- Ismail KA, Hawash YA, Saber T, Eed EM, Khalifa AS, Alsharif KF, Alghamdi SA, Khalifa AM, Khalifa OM, Althubiti HK, Alsofyani GM. 2020. Microsporidia infection in patients with autoimmune diseases. Indian Journal of Medical Microbiology, 38, 409–414. [CrossRef] [PubMed] [Google Scholar]
- Karim MR, Rume FI, Li D, Li J, Zhang L. 2022. First molecular characterization of Enterocytozoon bieneusi in children and calves in Bangladesh. Transboundary and Emerging Diseases, 69, 1999–2007. [CrossRef] [PubMed] [Google Scholar]
- Karimi K, Mirjalali H, Niyyati M, Haghighi A, Pourhoseingholi MA, Sharifdini M, Naderi N, Zali MR. 2020. Molecular epidemiology of Enterocytozoon bieneusi and Encephalitozoon sp., among immunocompromised and immunocompetent subjects in Iran. Microbial Pathogenesis, 141, 103988. [CrossRef] [PubMed] [Google Scholar]
- Kaya F, Inkaya AC, Aksoy S, Abbasoglu O, Ertenli AI, Buyukasik Y, Arikan AS, Akyon Y, Erguven S. 2021. Investigation of intestinal protozoon prevalence in immunocompromised patients at a university hospital. Türkiye Parazitoloji Dergisi, 45, 39–44. [CrossRef] [PubMed] [Google Scholar]
- Khanduja S, Ghoshal U, Agarwal V, Pant P, Ghoshal UC. 2017. Identification and genotyping of Enterocytozoon bieneusi among human immunodeficiency virus infected patients. Journal of Infection and Public Health, 10, 31–40. [CrossRef] [PubMed] [Google Scholar]
- Kicia M, Wesolowska M, Kopacz Z, Jakuszko K, Sak B, Kvetonova D, Krajewska M, Kvac M. 2016. Prevalence and molecular characteristics of urinary and intestinal microsporidia infections in renal transplant recipients. Clinical Microbiology and Infection, 22, 462–465. [Google Scholar]
- Lebbad M, Norrgren H, Naucler A, Dias F, Andersson S, Linder E. 2001. Intestinal parasites in HIV-2 associated AIDS cases with chronic diarrhoea in Guinea-Bissau. Acta Tropica, 80, 45–49. [CrossRef] [PubMed] [Google Scholar]
- Li W, Feng Y, Santin M. 2019. Host specificity of Enterocytozoon bieneusi and public health implications. Trends in Parasitology, 35, 436–451. [CrossRef] [PubMed] [Google Scholar]
- Li W, Feng Y, Xiao L. 2022. Enterocytozoon bieneusi. Trends in Parasitology, 38, 95–96. [CrossRef] [PubMed] [Google Scholar]
- Li W, Zhong Z, Song Y, Gong C, Deng L, Cao Y, Zhou Z, Cao X, Tian Y, Li H, Feng F, Zhang Y, Wang C, Li C, Yang H, Huang X, Fu H, Geng Y, Ren Z, Wu K, Peng G. 2018. Human-Pathogenic Enterocytozoon bieneusi in captive giant pandas (Ailuropoda melanoleuca) in China. Scientific Reports, 8, 6590. [CrossRef] [PubMed] [Google Scholar]
- Li X, Palmer R, Trout JM, Fayer R. 2003. Infectivity of microsporidia spores stored in water at environmental temperatures. Journal of Parasitology, 89, 185–188. [CrossRef] [PubMed] [Google Scholar]
- Liu H. 2015. Infection and genotype characteristic of the pathogens of enteric protozoan diseases and development of multiplex PCR method. CDC. (in Chinese). [Google Scholar]
- Liu H, Jiang Z, Yuan Z, Yin J, Wang Z, Yu B, Zhou D, Shen Y, Cao J. 2017. Infection by and genotype characteristics of Enterocytozoon bieneusi in HIV/AIDS patients from Guangxi Zhuang autonomous region, China. BMC Infectious Diseases, 17, 684. [CrossRef] [PubMed] [Google Scholar]
- Liu H, Shen Y, Yin J, Yuan Z, Jiang Y, Xu Y, Pan W, Hu Y, Cao J. 2014. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infectious Diseases, 14, 25. [CrossRef] [PubMed] [Google Scholar]
- Lobo ML, Augusto J, Antunes F, Ceita J, Xiao L, Codices V, Matos O. 2014. Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and other intestinal parasites in young children in Lobata province, Democratic Republic of Sao Tome and Principe. PLoS One, 9, e97708. [CrossRef] [PubMed] [Google Scholar]
- Lores B, Lopez-Miragaya I, Arias C, Fenoy S, Torres J, Del AC. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus – negative patients from Vigo, Spain. Clinical Infectious Diseases, 34, 918–921. [CrossRef] [PubMed] [Google Scholar]
- Lu-yao Q, Jing P, Wen-dao LI, Song-tao D, Han-deng L, Du HF. 2017. Investigation of the prevalence of Enterocytozoon bieneusi, Encephalitozoon intestinalis and Encephalitozoon cuniculi among diarrheal patients in the southwest of China. Chinese Journal of Mycology, 12, 257–261. [Google Scholar]
- Masoumi-Asl H, Khanaliha K, Bokharaei-Salim F, Esteghamati A, Kalantari S, Hosseinyrad M. 2019. Enteric opportunistic infection and the impact of antiretroviral therapy among HIV/AIDS patients from Tehran, Iran. Iranian Journal of Public Health, 48, 730–739. [PubMed] [Google Scholar]
- Matos O, Lobo ML, Xiao L. 2012. Epidemiology of Enterocytozoon bieneusi infection in humans. Journal of Parasitology Research, 2012, 981424. [CrossRef] [Google Scholar]
- Moniot M, Nourrisson C, Faure C, Delbac F, Favennec L, Dalle F, Garrouste C, Poirier P. 2021. Assessment of a multiplex PCR for the simultaneous diagnosis of intestinal cryptosporidiosis and microsporidiosis: epidemiologic report from a French prospective study. Journal of Molecular Diagnostics, 23, 417–423. [CrossRef] [Google Scholar]
- Muadica AS, Messa AE Jr, Dashti A, Balasegaram S, Santin M, Manjate F, Chirinda P, Garrine M, Vubil D, Acácio S, Köster PC, Bailo B, Nhampossa T, Calero-Bernal R, Mwenda JM, Mandomando I, Carmena D. 2020. First identification of genotypes of Enterocytozoon bieneusi (Microsporidia) among symptomatic and asymptomatic children in Mozambique. PLoS Neglected Tropical Diseases, 14, e8419. [Google Scholar]
- Muller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, Franzen C. 2001. Detection of microsporidia in travelers with diarrhea. Journal of Clinical Microbiology, 39, 1630–1632. [CrossRef] [PubMed] [Google Scholar]
- Naguib D, Roellig DM, Arafat N, Xiao L. 2022. Prevalence and genetic characterization of Enterocytozoon bieneusi in children in Northeast Egypt. Parasitology Research, 121, 2087–2092. [CrossRef] [PubMed] [Google Scholar]
- Ndzi ES, Asonganyi T, Nkinin MB, Xiao L, Didier ES, Bowers LC, Nkinin SW, Kaneshiro ES. 2016. Fast technology analysis enables identification of species and genotypes of latent microsporidia infections in healthy native Cameroonians. Journal of Eukaryotic Microbiology, 63, 146–152. [CrossRef] [PubMed] [Google Scholar]
- Ojuromi OT, Izquierdo F, Fenoy S, Fagbenro-Beyioku A, Oyibo W, Akanmu A, Odunukwe N, Henriques-Gil N, Del AC. 2012. Identification and characterization of microsporidia from fecal samples of HIV-positive patients from Lagos, Nigeria. PLoS One, 7, e35239. [CrossRef] [PubMed] [Google Scholar]
- Pagornrat W, Leelayoova S, Rangsin R, Tan-Ariya P, Naaglor T, Mungthin M. 2009. Carriage rate of Enterocytozoon bieneusi in an orphanage in Bangkok, Thailand. Journal of Clinical Microbiology, 47, 3739–3741. [CrossRef] [PubMed] [Google Scholar]
- Pavie J, Menotti J, Porcher R, Donay JL, Gallien S, Sarfati C, Derouin F, Molina JM. 2012. Prevalence of opportunistic intestinal parasitic infections among HIV-infected patients with low CD4 cells counts in France in the combination antiretroviral therapy era. International Journal of Infectious Diseases, 16, e677–e679. [CrossRef] [Google Scholar]
- Prasertbun R, Mori H, Pintong AR, Sanyanusin S, Popruk S, Komalamisra C, Changbunjong T, Buddhirongawatr R, Sukthana Y, Mahittikorn A. 2017. Zoonotic potential of Enterocytozoon genotypes in humans and pigs in Thailand. Veterinary Parasitology, 233, 73–79. [CrossRef] [PubMed] [Google Scholar]
- Prasertbun R, Mori H, Sukthana Y, Popruk S, Kusolsuk T, Hagiwara K, Mahittikorn A. 2019. Enterocytozoon bieneusi and Cryptosporidium: a cross-sectional study conducted throughout Thailand. BMC Infectious Diseases, 19, 808. [CrossRef] [PubMed] [Google Scholar]
- Qi M, Yu F, Zhao A, Zhang Y, Wei Z, Li D, Zhang L. 2020. Unusual dominant genotype NIA1 of Enterocytozoon bieneusi in children in Southern Xinjiang, China. PLoS Neglected Tropical Diseases, 14, e8293. [Google Scholar]
- Qin Y, Chen C, Qin Y, Yang X, Li M, Meng X, Zhao Z, Ma N, Cai Y, Zhang Y, Zhao Q. 2022. Prevalence and related factors of Enterocytozoon bieneusi in cattle: a global systematic review and meta-analysis. Preventive Veterinary Medicine, 208, 105775. [CrossRef] [PubMed] [Google Scholar]
- Qing L. 2018. Studies on prevalence and genotypes of G. lamblia, B. hominis and E. bieneusi in humans in Dali. Dali University. (in Chinese). [Google Scholar]
- Qiu L, Xia W, Li W, Ping J, Ding S, Liu H. 2019. The prevalence of microsporidia in China: a systematic review and meta-analysis. Scientific Reports, 28, 3174. [CrossRef] [PubMed] [Google Scholar]
- Rabeneck L, Gyorkey F, Genta RM, Gyorkey P, Foote LW, Risser JM. 1993. The role of microsporidia in the pathogenesis of HIV-related chronic diarrhea. Annals of Internal Medicine, 119, 859–895. [CrossRef] [PubMed] [Google Scholar]
- Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group. 2021. PRISMA-S: an extension to the PRISMA Statement for reporting literature searches in systematic reviews. Systematic Reviews, 10, 39. [CrossRef] [PubMed] [Google Scholar]
- Rong-Hua X, Gao-Xiang C, Shan-Shan OY. 2015. Analysis status of intestinal parasite infection among HIV/AIDS patients in Hengyang. Chinese Journal of Immunology, 31, 695–697. [Google Scholar]
- Ruan Y, Xu X, He Q, Li L, Guo J, Bao J, Pan G, Li T, Zhou Z. 2021. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasites & Vectors, 14, 186. [CrossRef] [PubMed] [Google Scholar]
- Sak B, Brady D, Pelikanova M, Kvetonova D, Rost M, Kostka M, Tolarova V, Huzova Z, Kvac M. 2011. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. Journal of Clinical Microbiology, 49, 1064–1070. [CrossRef] [PubMed] [Google Scholar]
- Sak B, Kucerova Z, Kvac M, Kvetonova D, Rost M, Secor EW. 2010. Seropositivity for Enterocytozoon bieneusi, Czech Republic. Emerging Infectious Diseases, 16, 335–337. [CrossRef] [PubMed] [Google Scholar]
- Sak B, Kvac M, Kucerova Z, Kvetonova D, Sakova K. 2011. Latent microsporidial infection in immunocompetent individuals – A longitudinal study. PLoS Neglected Tropical Diseases, 5, e1162. [CrossRef] [PubMed] [Google Scholar]
- Samie A, Maluleke RP, Tanih N, ElBakri A. 2021. Molecular epidemiology of microsporidia among HIV-positive and HIV-negative patients in the Limpopo province, South Africa. Journal of Infection in Developing Countries, 15, 710–718. [CrossRef] [PubMed] [Google Scholar]
- Sander VA, Sánchez López EF, Mendoza Morales L, Ramos Duarte VA, Corigliano MG, Clemente M. 2020. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Frontiers in Cellular and Infection Microbiology, 10, 288. [CrossRef] [PubMed] [Google Scholar]
- Santin M, Fayer R. 2011. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Research in Veterinary Science, 90, 363–371. [CrossRef] [PubMed] [Google Scholar]
- Seatamanoch N, Kongdachalert S, Sunantaraporn S, Siriyasatien P, Brownell N. 2022. Microsporidia, a highly adaptive organism and its host expansion to humans. Frontiers in Cellular and Infection Microbiology, 12, 924007. [CrossRef] [PubMed] [Google Scholar]
- Shahrul Anuar T, Al-Mekhlafi HM, Md Salleh F, Moktar N. 2013. New insights of microsporidial infection among asymptomatic aboriginal population in Malaysia. PLoS One, 8, e71870. [CrossRef] [PubMed] [Google Scholar]
- Shehab AY, Moneer EA, Allam AF, Khalil SS, Tolba MM. 2021. Intestinal microsporidia infection in leukemic children: microscopic and molecular detection. Acta Parasitologica, 66, 346–353. [CrossRef] [PubMed] [Google Scholar]
- Shen Y, Gong B, Liu X, Wu Y, Yang F, Xu J, Zhang X, Cao J, Liu A. 2020. First identification and genotyping of Enterocytozoon bieneusi in humans in Myanmar. BMC Microbiology, 20, 10. [CrossRef] [PubMed] [Google Scholar]
- Sokolova OI, Demyanov AV, Bowers LC, Didier ES, Yakovlev AV, Skarlato SO, Sokolova YY. 2011. Emerging microsporidian infections in Russian HIV-infected patients. Journal of Clinical Microbiology, 49, 2102–2108. [CrossRef] [PubMed] [Google Scholar]
- Sulaiman IM, Bern C, Gilman R, Cama V, Kawai V, Vargas D, Ticona E, Vivar A, Xiao L. 2003. A molecular biologic study of Enterocytozoon bieneusi in HIV-infected patients in Lima, Peru. Journal of Eukaryotic Microbiology, 50(Suppl), 591–596. [CrossRef] [PubMed] [Google Scholar]
- Sutthikornchai C, Popruk S, Mahittikorn A, Arthan D, Soonthornworasiri N, Paratthakonkun C, Feng Y, Xiao L. 2021. Molecular detection of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi in school children at the Thai-Myanmar border. Parasitology Research, 120, 2887–2895. [CrossRef] [PubMed] [Google Scholar]
- Tao WF, Ni HB, Du HF, Jiang J, Li J, Qiu HY, Ye-Li, Zhang XX. 2020. Molecular detection of Cryptosporidium and Enterocytozoon bieneusi in dairy calves and sika deer in four provinces in Northern China. Parasitology Research, 119, 105–114. [CrossRef] [PubMed] [Google Scholar]
- Tuli L, Singh DK, Gulati AK, Sundar S, Mohapatra TM. 2010. A multiattribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patients. BMC Microbiology, 10, 11. [CrossRef] [PubMed] [Google Scholar]
- Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Buckholt MA, Tzipori S. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. American Journal of Tropical Medicine and Hygiene, 67, 299–303. [Google Scholar]
- Udonsom R, Prasertbun R, Mahittikorn A, Chiabchalard R, Sutthikornchai C, Palasuwan A, Popruk S. 2019. Identification of Enterocytozoon bieneusi in goats and cattle in Thailand. BMC Veterinary Research, 15, 308. [CrossRef] [PubMed] [Google Scholar]
- van Gool T, Dankert J. 1995. Human microsporidiosis: clinical, diagnostic and therapeutic aspects of an increasing infection. Clinical Microbiology and Infection, 1, 75–85. [CrossRef] [PubMed] [Google Scholar]
- van Gool T, Luderhoff E, Nathoo KJ, Kiire CF, Dankert J, Mason PR. 1995. High prevalence of Enterocytozoon bieneusi infections among HIV-positive individuals with persistent diarrhoea in Harare, Zimbabwe. Transactions of the Royal Society of Tropical Medicine and Hygiene, 89, 478–480. [CrossRef] [PubMed] [Google Scholar]
- Velasquez JN, di Risio C, Etchart C, Chertcoff AV, Astudillo OG, Carnevale S. 2019. Multimethodological approach to gastrointestinal microsporidiosis in HIV-infected patients. Acta Parasitologica, 64, 658–669. [CrossRef] [PubMed] [Google Scholar]
- Wang L, Lihua X, Liping D, Ye J, Guo Y, Guo M, Lili L, Yaoyu F. 2013. Concurrent Infections of Giardia duodenalis, Enterocytozoon bieneusi, and Clostridium difficile in children during a cryptosporidiosis outbreak in a pediatric hospital in China. PLOS Neglected Tropical Diseases, 7, e2437. [CrossRef] [PubMed] [Google Scholar]
- Wang L, Zhang H, Zhao X, Zhang L, Zhang G, Guo M, Liu L, Feng Y, Xiao L. 2013. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. Journal of Clinical Microbiology, 51, 557–563. [CrossRef] [PubMed] [Google Scholar]
- Wang T, Fan Y, Koehler AV, Ma G, Li T, Hu M, Gasser RB. 2017. First survey of Cryptosporidium, Giardia and Enterocytozoon in diarrhoeic children from Wuhan, China. Infection Genetics and Evolution, 51, 127–131. [CrossRef] [Google Scholar]
- Wuhib T, Silva TM, Newman RD, Garcia LS, Pereira ML, Chaves CS, Wahlquist SP, Bryan RT, Guerrant RL, Sousa AQ, de Queiroz TRBS, Sears CL. 1994. Cryptosporidial and microsporidial infections in human immunodeficiency virus-infected patients in northeastern Brazil. Journal of Infectious Diseases, 170, 494–497. [CrossRef] [PubMed] [Google Scholar]
- Xu C, Ma X, Zhang H, Zhang XX, Zhao JP, Ba HX, Rui-Du Xing XM, Wang QK, Zhao Q. 2016. Prevalence, risk factors and molecular characterization of Enterocytozoon bieneusi in raccoon dogs (Nyctereutes procyonoides) in five provinces of Northern China. Acta Tropica, 161, 68–72. [CrossRef] [PubMed] [Google Scholar]
- Xu L. 2011. Epidemiological Investigation of microsporidias and molecular characteristics of microsproridia isolation in Zhengzhou, Henan Province. Henan Agricultural University. (in Chinese). [Google Scholar]
- Xu N. 2019. Molecular epidemiology of intestinal protozoa in some rural areas of Hunan Province and prediction of Cryptosporidium infection in Binyang, Guangxi. CDC. (in Chinese). [Google Scholar]
- Yamashiro S, Fiuza V, Teixeira A, Branco N, Levy CE, Castro I, Franco R. 2017. Enterocytozoon bieneusi detected by molecular methods in raw sewage and treated effluent from a combined system in Brazil. Memorias do Instituto Oswaldo Cruz, 112, 403–410. [CrossRef] [PubMed] [Google Scholar]
- Yang J, Song M, Wan Q, Li Y, Lu Y, Jiang Y, Tao W, Li W. 2014. Enterocytozoon bieneusi genotypes in children in Northeast China and assessment of risk of zoonotic transmission. Journal of Clinical Microbiology, 52, 4363–4367. [CrossRef] [PubMed] [Google Scholar]
- Yu F, Li D, Chang Y, Wu Y, Guo Z, Jia L, Xu J, Li J, Qi M, Wang R, Zhang L. 2019. Molecular characterization of three intestinal protozoans in hospitalized children with different disease backgrounds in Zhengzhou, central China. Parasites & Vectors, 12, 543. [CrossRef] [PubMed] [Google Scholar]
- Zajaczkowska Z, Akutko K, Kvac M, Sak B, Szydlowicz M, Hendrich AB, Iwanczak B, Kicia M. 2021. Enterocytozoon Bieneusi infects children with inflammatory bowel disease undergoing immunosuppressive treatment. Frontiers in Medicine (Lausanne), 8, 741–751. [Google Scholar]
- Zhang N, Wu R, Ji T, Cui LL, Cao HX, Li D, Li J, Zhang L, Huang C, Zhou DH. 2020. Molecular detection, multilocus genotyping, and population genetics of Enterocytozoon bieneusi in pigs in Southeastern China. Journal of Eukaryotic Microbiology, 67, 107–114. [CrossRef] [PubMed] [Google Scholar]
- Zhang T, Ren G, Zhou H, Qiang Y, Li J, Zhang Y, Li T, Zhou Y, Wang Y, Lai X, Lei S, Tan F, Liu R, Li W, He J, Zhao W, Zhu C, Lu G. 2022. Molecular prevalence and genetic diversity analysis of Enterocytozoon bieneusi in humans in Hainan Province, China: high diversity and unique endemic genetic characteristics. Frontiers in Public Health, 10, 1007130. [CrossRef] [PubMed] [Google Scholar]
- Zhang X, Wang Z, Su Y, Liang X, Sun X, Peng S, Lu H, Jiang N, Yin J, Xiang M, Chen Q. 2011. Identification and Genotyping of Enterocytozoon bieneusi in China. Journal of Clinical Microbiology, 49, 2006–2008. [CrossRef] [PubMed] [Google Scholar]
- Zhang Y, Koehler AV, Wang T, Gasser RB. 2021. Enterocytozoon bieneusi of animals – with an “Australian twist”. Advances in Parasitology, 111, 1–73. [CrossRef] [PubMed] [Google Scholar]
- Zhang Y, Koehler AV, Wang T, Robertson GJ, Bradbury RS, Gasser RB. 2018. Enterocytozoon bieneusi genotypes in people with gastrointestinal disorders in Queensland and Western Australia. Infection Genetics and Evolution, 65, 293–299. [CrossRef] [Google Scholar]
- Zhou L, Guan Z, Chen C, Zhu Q, Qiu S, Liu Y, Li M, Zeng W, Wang H, Gao Y, Yuan Y, Zhang H, Ruan G, Pan X. 2022. The successful treatment of Enterocytozoon bieneusi microsporidiosis with nitazoxanide in a patient with B-ALL: a case report. Frontiers in Cellular and Infection Microbiology, 12, 107–2463. [Google Scholar]
Cite this article as: Wang Y, Li X-M, Yang X, Wang X-Y, Wei Y-J, Cai Y, Geng H-L, Yang X-B, Yu H-L, Jiang J & Cao H. 2024. Global prevalence and risk factors of Enterocytozoon bieneusi infection in humans: a systematic review and meta-analysis. Parasite 31, 9.
All Tables
Summary of global E. bieneusi human infection rates and relevant characteristics.
Prevalence estimates of E. bieneusi infection, and estimated numbers of infected people in 34 countries.
All Figures
 |
Figure 1 Flow diagram of literature search and selection. |
| In the text | |
 |
Figure 2 Forest map of global human infection in E. bieneusi. |
| In the text | |
 |
Figure 3 Map of E. bieneusi prevalence in human worldwide. |
| In the text | |
 |
Figure 4 Evolutionary tree of E. bieneusi prevalence in human worldwide. |
| In the text | |
 |
Figure 5 Prevalence of E. bieneusi genotype in different countries. |
| In the text | |
 |
Figure S1: Funnel plot with pseudo 95% confidence interval for publication bias test. |
| In the text | |
 |
Figure S2: Egger’s test for publication bias. |
| In the text | |
 |
Figure S3: Shear complement graph and pseudo 95% confidence interval for publication bias test. |
| In the text | |
 |
Figure S4: Figure S4: Sensitivity analysis of human infection with E. bieneusi. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


