| Issue |
Parasite
Volume 27, 2020
|
|
|---|---|---|
| Article Number | 11 | |
| Number of page(s) | 5 | |
| DOI | https://doi.org/10.1051/parasite/2020008 | |
| Published online | 19 February 2020 | |
Research Article
Seroprevalence of Toxoplasma gondii infection in sheep in Inner Mongolia Province, China
Séroprévalence de l’infection par Toxoplasma gondii des moutons dans la province de Mongolie intérieure, Chine
1
Food Science and Engineering College of Inner Mongolia Agricultural University, Hohhot 010018, PR China
2
Inner Mongolia Food Safety and Inspection Testing Center, Hohhot 010090, PR China
3
Inner Mongolia KingGoal Technology Service Co., Ltd., Hohhot 010010, PR China
* Corresponding author: yanxinlei1987620@foxmail.com
Received:
6
January
2020
Accepted:
8
February
2020
Toxoplasma gondii is an important zoonotic parasite that can infect almost all warm-blooded animals, including humans, and infection may result in many adverse effects on animal husbandry production. Animal husbandry in Inner Mongolia is well developed, but data on T. gondii infection in sheep are lacking. In this study, we determined the seroprevalence and risk factors associated with the seroprevalence of T. gondii using an indirect enzyme-linked immunosorbent assay (ELISA) test. A total of 1853 serum samples were collected from 29 counties of Xilin Gol League (n = 624), Hohhot City (n = 225), Ordos City (n = 158), Wulanchabu City (n = 144), Bayan Nur City (n = 114) and Hulunbeir City (n = 588). The overall seroprevalence of T. gondii was 15.43%. Risk factor analysis showed that seroprevalence was higher in sheep ≥12 months of age (21.85%) than that in sheep <12 months of age (10.20%) (p < 0.01). Seroprevalence was higher in male sheep (18.76%) than females (12.80%) (p < 0.01). Barn-feeding sheep (23.13%) had higher prevalence than grazing sheep (10.94%) (p < 0.01). The seroprevalence was significantly different in different districts (p < 0.01). This study shows that sheep are exposed to T. gondii in Inner Mongolia, and provides a data reference for public health and disease control.
Résumé
Toxoplasma gondii est un parasite zoonotique important qui peut infecter presque tous les animaux à sang chaud, y compris les humains, et son infection peut entraîner de nombreux effets néfastes sur la production animale. L’élevage de Mongolie intérieure est développé, mais les données sur l’infection des moutons par T. gondii manquent. Dans cette étude, nous avons déterminé la séroprévalence et les facteurs de risque associés à la séroprévalence de T. gondii en utilisant un test immuno-enzymatique indirect (ELISA). Un total de 1853 échantillons de sérum ont été prélevés dans vingt-neuf comtés de la Ligue Xilin Gol (n = 624), Hohhot City (n = 225), Ordos City (n = 158), Wulanchabu City (n = 144), Bayan Nur City (n = 114) et la ville de Hulunbeir (n = 588). La séroprévalence globale de T. gondii était de 15,43 %. L’analyse des facteurs de risque a montré que le taux d’infection était plus élevé chez les ovins ≥ 12 mois (21,85 %) que chez les ovins <12 mois (10,20 %) (p < 0,01). Les moutons mâles (18,76 %) avaient une séroprévalence plus élevée que les femelles (12,80 %) (p < 0,01). Les moutons nourris à l’étable (23,13 %) avaient une séroprévalence plus élevée que ceux au pâturage (10,94 %) (p < 0,01). La séroprévalence était significativement différente entre les différents districts (p < 0,01). L’étude a montré que les moutons étaient exposés à T. gondii en Mongolie intérieure et a fourni une référence de données pour la santé publique et la lutte contre les maladies.
Key words: Toxoplasma gondii / ELISA / Seroprevalence / Sheep / Inner Mongolia / China
© X. Yan et al., published by EDP Sciences, 2020
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Toxoplasma gondii is a food-borne intracellular parasite that can infect nearly all warm-blooded animals worldwide, even humans [9]. Approximately one-third of the human population has been exposed to T. gondii. Infection by the parasite may cause cerebral and ocular damage and even death, especially in immunodeficient patients [8, 18]. Humans are mainly infected with T. gondii by ingesting uncooked meat and water contaminated by oocysts from the environment, or by vertical transmission [2, 3, 7]. In addition, T. gondii can also have a negative influence on animal growth, development and reproduction, and cause great economic loss to livestock husbandry [6]. Livestock become infected mainly by ingesting food and water contaminated with sporulated oocysts [12]. Toxoplasma gondii infection in sheep can cause a wide variety of non-specific symptoms (fever and dyspnoea), and specific symptoms (depression, lethargy, vomiting, diarrhea, chorioretinitis, and lymphadenopathy), and can even cause abortions and stillbirths [10]. Recently, increasing consumption of mutton has raised the risk of T. gondii infection. Sero-epidemiological surveys have reported a global distribution of T. gondii in sheep ranging from less than 4.4% to over 80.0% [5, 14, 19].
As a major livestock husbandry Province in China, the number of sheep stocks in Inner Mongolia has reached over 100 million in recent years, and the animal production economy is one of the most important pillar industries in this district. In the past, many studies have examined the seroprevalence of T. gondii infection in livestock, including horses and cattle in Inner Mongolia [16, 21]. However, data on the seroprevalence of T. gondii in sheep in Inner Mongolia are not comprehensive nor detailed [4]. The natural grassland of Xilin Gol League and Hulunbeir City is famous throughout the world for its high quality, and sheep production here is prosperous. The population in Hohhot City, Ordos City, Wulanchabu City, and Bayan Nur City accounts for half of the total population in Inner Mongolia. Therefore, samples were collected from these areas, which makes the results more representative. The aim of this study was to determine the current status of the prevalence of T. gondii in sheep in Inner Mongolia, and to provide a reference for the prevention and control of T. gondii. The results will serve as baseline comparison data for future industrial development and safety assessments, and provide information to public health departments, wildlife managers and researchers.
Materials and methods
Serum samples
Blood samples were collected from 29 counties of Xilin Gol League, Hohhot City, Ordos City, Wulanchabu City, Bayan Nur City, and Hulunbeir City (Fig. 1) to investigate the presence of serum antibodies against T. gondii. A total of 1853 blood samples were selected randomly from September 2018 to November 2019, and the background information of each sample was obtained mainly from the hosts or breeders. No special criteria were applied to different farms. During visits to the localities, blood samples were collected from the jugular vein into a centrifuge tube. These centrifuge tubes filled with blood samples were quickly frozen in a local freezer once collected, and brought back to the laboratory in an incubator. Each of the blood samples was centrifuged at 4000 rpm for 8 min, and serum was separated and stored at −20 °C until further analysis.
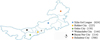 |
Figure 1 Blood samples were collected from 29 counties of Xilin Gol League, Hohhot City, Ordos City, Wulanchabu City, Bayan Nur City, and Hulunbeir City. |
Determination of antibodies against T. gondii
Antibodies against T. gondii from serum samples were detected by an indirect enzyme-linked immunosorbent assay (ELISA) test, using a commercially available kit (CK-DN74810, 96T), which was obtained from Quanzhou Ruixin Biotechnology Co., Ltd. The detection procedure was carried out in accordance with the protocol described by the manufacturer. When the reaction was complete, the optical density (OD) value was measured at 450 nm using a Microplate Reader within 15 min. Positive and negative controls provided within the kit were included in each test. The serum samples were considered positive if the sample OD value was greater than the cut-off (the cut-off was the sum of the average value of the negative control OD value and 0.15).
Statistical analysis
Statistical analysis was carried out by chi-square (χ2) testing with SPSS (Statistical Analysis System, Version 20.0). When p < 0.01, the difference was considered extremely significant; when 0.01 < p < 0.05, the difference was considered significant; when p > 0.05, the difference was not significant. The odds ratio (OR) at the 95% confidence level was used for the determinants influencing the epidemiology of parasites.
Results
Antibodies against T. gondii were found in 286 of the 1853 sheep by the ELISA kit (Table 1); the overall seroprevalence was 15.43%. On the basis of values for T. gondii antibody detection, the seroprevalence of four districts was higher than the overall seroprevalence, and Bayan Nur City had the highest seroprevalence (23.68%). Across all districts, Xilin Gol League had the lowest seroprevalence (12.02%). Chi-square test analysis showed that there were significant differences in the prevalence of T. gondii infection in different districts (χ2 = 112.010, p value = 0.000) (Table 1).
Prevalence of T. gondii infection in different districts by ELISA.
In this study, 154 of the 821 male sheep serum samples tested were positive, with a positive rate of 18.76%, and 132 of the 1032 female sheep serum samples tested were positive, with a positive rate of 12.80% (Table 2). There was a significant difference in the seroprevalence of T. gondii infection between the sexes (p < 0.01). Sheep ≥12 months of age were at higher risk (21.85%, 182/833) than sheep <12 months (10.20%, 104/1020). Barn-feeding sheep were at higher risk (23.13%, 158/683) than grazing sheep (10.94%, 128/1070). There were significant differences in the seroprevalence of T. gondii infection in different ages and rearing models (p < 0.01). Risk factor analyses showed that sex (OR = 0.682), age (OR = 0.467), and rearing model (OR = 0.473) were risk factors for T. gondii infection in sheep (Table 2).
Prevalence of T. gondii infection in different sexes, ages, and rearing models by ELISA.
Over a period of one year, we collected blood samples from different districts every month. Compared with other months, October had the highest prevalence (21.69%, 41/189), and February had the lowest prevalence (10.74%, 13/121). Chi-square test analysis showed that there was no significant difference in the seroprevalence of T. gondii infection in different months (χ2 = 23.157, p value = 0.393) (Table 3).
Seroprevalence of T. gondii infection in different months by ELISA.
Discussion
Antibodies against T. gondii were found in 286 out of 1853 sheep (15.43%) in this study, which was higher than that reported in Shandong in 2019 (9.84%) and Yunnan in 2015 (9.70%) [1, 22]. Moreover, Gao et al. reported a prevalence of 17.10% (13/76) for T. gondii infection in Chifeng, Inner Mongolia, which was in the same range as the prevalence of infection in this study [4]. In this study, the seroprevalence of T. gondii in sheep varied from 5.00% to 29.63% among different counties. There was a great difference in the prevalence of T. gondii infection in different districts. We speculated that many factors contributed to this difference, such as climate, elevation, sheep strain, feeding model, and level of disease prevention and control, bearing in mind the vast size of Inner Mongolia Province (1.18 million km2). Therefore, in order to make the data more accurate, samples will be collected from more districts in the future.
Moreover, there were significant differences in the seroprevalence of T. gondii by sex. Males (18.76%) had a higher risk than females (12.80%) (p < 0.01). However, studies in Henan, China, revealed a higher prevalence of T. gondii in females than in males [20]. Studies in Yunan, China found no association between sex and the prevalence of T. gondii [22]. According to Romanelli et al. the presence of oestrogen in females normally increases immunity, and androgen in males decreases immunity [13]. Therefore, we suspect that sex is likely to work in conjunction with other unknown factors. Moreover, we found that the seroprevalence of T. gondii infection had a significant difference concerning age (sheep ≥12 months: 21.85%, and sheep <12 months: 10.20%). A total of 10.20% (104/1020) of sheep <12 months were seropositive. The higher prevalence in sheep ≥12 months was likely due to the prolonged time of exposure and repeated exposure to the oocyst-contaminated environment, resulting in a greater possibility of infection. In this study, there were significant differences in the seroprevalence between barn feeding and grazing rearing systems. Other studies have also shown that barn feeding involves a higher risk than grazing [11]. Since grazing sheep are pastured in comparatively large grazing areas, these sheep are exposed to T. gondii oocysts at a low level. However, barn feeding sheep were raised in a concentrated manner, which may increase the chances of T. gondii infection among sheep once food, water, or the environment was contaminated by oocysts. This may be the reason why the T. gondii infection prevalence in Bayan Nur City was the highest in our study (barn feeding sheep: 114, and no grazing sheep). At the same time, there was no significant difference in the seroprevalence in different months. However, some studies have shown that the seroprevalence is probably related to seasons [17], and the reason for this difference may be due to different environments, temperatures, and various sample qualities. Moreover, studies have shown that high temperatures have little impact on the reduction in viability of T. gondii [15].
In this study, seropositive samples were found in 29 counties of all six districts, which suggested that T. gondii infection was common in sheep in Inner Mongolia. As an important foodborne zoonotic parasite, T. gondii is seriously harmful to people and animals with various routes of infection. Therefore, great attention should be paid to the prevention and control of T. gondii in sheep. Certain measures can be taken to reduce the prevalence of T. gondii infection in sheep, such as strengthening the management of sheep farms, keeping the barn clean, and preventing feline excreta from polluting sheepfolds, food, or drinking water.
Conflict of interest
All individual authors declare that they have no conflict of interest (financial, personal, or other).
Acknowledgments
This work was financially supported by Research project of high level talents in Inner Mongolia Agricultural University (No. RZ1900002817) and the Program of Inner Mongolia Natural Science Foundation of China (No. 2018BS03015).
References
- Ai K, Huang CH, Guo JJ, Cong H, He SH, Zhou CX, Cong W. 2019. Molecular detection of Toxoplasma gondii in the slaughter sheep and goats from Shandong Province, Eastern China. Vector-Borne and Zoonotic Diseases, 2488–2491. DOI: 10.1089/vbz.2019.2488. [Google Scholar]
- Cong W, Chen L, Shan XF. 2018. First genetic characterization of Toxoplasma gondii infection in donkey meat slaughtered for human consumption in Shandong Province, Eastern China. Infection Genetics and Evolution, 61, 1–3. [CrossRef] [Google Scholar]
- Dubey JP, Darrington C, Tiao N, Ferreira LR, Choudhary S, Molla B, Saville WJA, Tilahun G, Kwok OCH, Gebreyes WA. 2013. Isolation of viable Toxoplasma gondii from tissues and feces of cats from Addis Ababa, Ethiopia. Journal of Parasitology, 99(1), 56–58. [CrossRef] [Google Scholar]
- Gao Y, Guo HP, Moumouni APF, Sun M, Liu MM, Efstratiou A, Lee SH, Wang GB, Li JX, Li YC. 2018. Seroprevalence of Toxoplasma gondii infection in sheep from Northern China. Tropical Biomedicine, 35(3), 664–668. [Google Scholar]
- Gazzonis A, Villa L, Manfredi MT, Zanzani S. 2019. Spatial analysis of infections by Toxoplasma gondii and Neospora caninum (Protozoa: Apicomplexa) in small ruminants in Northern Italy. Animals, 9(11), 1–14. [CrossRef] [Google Scholar]
- Gos ML, Manazza JA, Späth EJA, Pardini L, Fiorentino MA, Unzaga JM, Moré G, Venturini MC. 2017. Seroprevalence of toxoplasma gondii and Neospora caninum infections in goats from two Argentinean Provinces (Article). Open Veterinary Journal, 7(4), 319–322. [CrossRef] [PubMed] [Google Scholar]
- Heddergott M, Frantz AC, Stubbe M, Stubbe A, Ansorge H, Osten-Sacken N. 2017. Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central Europe (Article). Parasitology Research, 8, 2335–2340. [Google Scholar]
- Lalle M, Possenti A, Pozio E, Dubey JP. 2018. Loop-mediated isothermal amplification – lateral – flow dipstick (LAMP – LFD) to detect Toxoplasma gondii oocyst in ready – to – eat salad. Food Microbiology, 70, 137–142. [CrossRef] [PubMed] [Google Scholar]
- Melo RPB, Almeida JC, de Lima DCV, Carvalho JCS, Porto WJN, Magalhaes FJR, Hamilton CM, Katzer F, Mota RA. 2019. Atypical Toxoplasma gondii genotype from a sheep and a pig on Fernando de Noronha Island, Brazil, showed different mouse virulence profiles. Parasitology Research, 119, 351–356. [CrossRef] [PubMed] [Google Scholar]
- Mohamed-Cherif A, Miroud K, Benfodil K, Ansel S, Khelef D, Kaidi R, Ait-Oudhia K. 2019. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep, and goats in Algeria: seroprevalence and risk factors. Veterinary Sciences, 6(3), 63–77. [Google Scholar]
- Nikolaos T, Pavlo M, Franz JC, Evaggelos K, Christos B, Smaragda S, Gereon S. 2012. Toxoplasma gondii in sheep and goats: seroprevalence and potential risk factors under dairy husbandry practices. Veterinary Parasitology, 190(3–4), 340–348. [CrossRef] [PubMed] [Google Scholar]
- Pablos-Tanarro A, Ortega-Mora LM, Palomo A, Casasola F, Ferre I. 2018. Seroprevalence of Toxoplasma gondii in Iberian pig sows. Parasitology Research, 117(5), 1419–1424. [CrossRef] [PubMed] [Google Scholar]
- Romanelli PR, Freire RL, Vidotto O, Marana ERM, Ogawa L, De Paula VSO, Garcia JL, Navarro IT. 2017. Prevalence of Neospora caninum and Toxoplasma gondii in sheep and dogs from Guarapuava farms, Paraná State, Brazil. Research in Veterinary Science, 82(2), 202–207. [Google Scholar]
- Rouatbi M, Amairia S, Lahmer M, Lassoued N, Rekik M, Wieland B, Mwacharo JM, Gharbi M. 2019. Detection of Toxoplasma gondii infection in semen of rams used for natural mating in commercial sheep farms in Tunisia. Veterinary Parasitology: Regional Studies and Reports, 18, 100341. [CrossRef] [Google Scholar]
- Rousseau A, Villena I, Dumetre A, Escotte-Binet S, Favennec L, Dubey JP, Aubert D, La Carbona S. 2019. Evaluation of propidium monoazide-based qPCR to detect viable oocysts of Toxoplasma gondii. Parasitology Research, 118(3), 999–1010. [CrossRef] [PubMed] [Google Scholar]
- Sun WW, Meng QF, Cong W, Shan XF, Wang CF, Qian AD. 2015. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitology Research, 114(11), 4211–4218. [CrossRef] [PubMed] [Google Scholar]
- Wang YG, Gui BZ, Li RC, Wang GP, Ge M, Liu GH. 2019. Seroprevalence and risk factors of Toxoplasma gondii infection in growth stages of pigs in Hunan Province, Subtropical China. Vector Borne and Zoonotic Diseases, 12, 945–949. [CrossRef] [Google Scholar]
- Webster JP. 2010. Dubey, J.P. Toxoplasmosis of animals and humans. Parasites & Vectors, 3(1), 112–113. [Google Scholar]
- Yang N, Li H, He J, Mu MY, Yang SH. 2013. Seroprevalence of Toxoplasma gondii infection in domestic sheep in Liaoning Province, Northeastern China. Journal of Parasitology, 99(1), 174–175. [CrossRef] [Google Scholar]
- Zhang N, Wang S, Wang D, Li CY, Zhang ZC, Yao ZJ, Li TT, Xie Q, Liu SG, Zhang HZ. 2016. Seroprevalence of Toxoplasma gondii infection and risk factors in domestic sheep in Henan Province, Central China. Parasite, 23, 53. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Zhang XX, Ren WX, Hou G, Liu Q, Yu TQ, Zhao Q, Ni HB. 2018. Seroprevalence and risk factors of Toxoplasma gondii infection in horses in Jilin Province and Inner Mongolia Autonomous Region, Northern China. Acta Tropica, 187, 119–123. [CrossRef] [PubMed] [Google Scholar]
- Zou FC, Yu X, Yang Y, Hu S, Chang H, Yang JF, Duan G. 2015. Seroprevalence and risk factors of Toxoplasma gondii infection in buffaloes, sheep and goats in Yunnan Province, Southwestern China. Iranian Journal of Parasitology, 10(4), 648–651. [PubMed] [Google Scholar]
Cite this article as: Yan X, Han W, Wang Y, Zhang H & Gao Z. 2020. Seroprevalence of Toxoplasma gondii infection in sheep in Inner Mongolia Province, China. Parasite 27, 11.
All Tables
Prevalence of T. gondii infection in different sexes, ages, and rearing models by ELISA.
All Figures
 |
Figure 1 Blood samples were collected from 29 counties of Xilin Gol League, Hohhot City, Ordos City, Wulanchabu City, Bayan Nur City, and Hulunbeir City. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


