| Issue |
Parasite
Volume 20, 2013
|
|
|---|---|---|
| Article Number | 56 | |
| Number of page(s) | 26 | |
| DOI | https://doi.org/10.1051/parasite/2013055 | |
| Published online | 19 December 2013 | |
urn:lsid:zoobank.org:pub:57C9DB24-D0A5-4730-93BE-1057D4419409
Research Article
Bucephalidae (Digenea) from epinephelines (Serranidae: Perciformes) from the waters off New Caledonia, including Neidhartia lochepintade n. sp.
Bucephalidae (Digenea) d’Epinephelinae (Serranidae: Perciformes) en Nouvelle-Calédonie, y compris Neidhartia lochepintade n. sp.
1
Department of Zoology, Natural History Museum, Cromwell Road, London
SW7 5BD, UK
2
UMR 7138, Systématique, Adaptation, Évolution, Muséum National d’Histoire Naturelle, Case postale 51, 55 rue Buffon, 75231
Paris Cedex 05, France
* Corresponding author: rab@nhm.ac.uk
Received:
7
October
2013
Accepted:
4
December
2013
Many bucephalid species, mainly of the subfamily Prosorhynchinae, have been described from epinepheline serranids (groupers) throughout the World’s Oceans. In this paper eight named prosorhynchine species are described and/or illustrated from epinepheline fishes from New Caledonia. Neidhartia lochepintade n. sp. in Epinephelus chlorostigma differs from other Neidhartia spp. in various combinations of distinct body-size, rhynchus size, previtelline and pre-mouth distance, post-testicular distance, cirrus-sac reach and egg-size. Other species are: Neidhartia haywardi Bott, Miller & Cribb, 2013 in Plectropomus leopardus; Neidhartia tyleri Bott, Miller & Cribb, 2013 in Plectropomus leopardus and Plectropomus laevis; Prosorhynchus freitasi Nagaty, 1937 in Plectropomus leopardus and Plectropomus laevis; Prosorhynchus robertsthomsoni Bott & Cribb, 2009 in Cephalopholis argus; Prosorhynchus longisaccatus Durio & Manter, 1968 in Cephalopholis urodeta, Epinephelus areolatus, Epinephelus cyanopodus and Epinephelus maculatus. Prosorhynchus luzonicus Velasquez, 1959 and Prosorhynchus sp. B. in Epinephelus coioides; Prosorhynchus serrani Durio & Manter, 1968 in Variola albimarginata and Variola louti; Prosorhynchus sp. A in Epinephelus morrhua; Prosorhynchus sp. immature in Epinephelus coeruleopunctatus. The new combination Neidhartia longivesicula (Bilqees, Khalil, Khan, Perveen & Muti-ur-Rehman, 2009) (Syn. Prosorhynchus longivesicula) is formed. Evidence from this paper and earlier molecular studies indicates that there are numerous morphologically similar prosorhynchine species in serranids, most of which show a high degree of host-specificity.
Résumé
De nombreuses espèces de Bucephalidae, principalement de la sous-famille Prosorhynchinae, ont été décrites de Serranidae Epinephelinae (mérous) à travers les océans du monde. Dans cet article, huit espèces nommées de Prosorhynchinae sont décrites et / ou illustrées d’Epinephelinae de Nouvelle-Calédonie. Neidhartia lochepintade n. sp., parasite d’Epinephelus chlorostigma, diffère des autres espèces de Neidhartia par des combinaisons variées de taille du corps, taille du rhynchus, distance pré-vitelline et pré-bouche, distance post-testiculaire, étendue du sac du cirre et taille des œufs. Les autres espèces sont : Neidhartia haywardi Bott, Miller & Cribb, 2013 chez Plectropomus leopardus; Neidhartia tyleri Bott, Miller & Cribb, 2013 chez Plectropomus leopardus et Plectropomus laevis; Prosorhynchus freitasi Nagaty, 1937 chez Plectropomus leopardus et Plectropomus laevis; Prosorhynchus robertsthomsoni Bott & Cribb, 2009 chez Cephalopholis argus; Prosorhynchus longisaccatus Durio & Manter, 1968 chez Cephalopholis urodeta, Epinephelus areolatus, Epinephelus cyanopodus et Epinephelus maculatus; Prosorhynchus luzonicus Velasquez, 1959 et Prosorhynchus sp. B. chez Epinephelus coioides; Prosorhynchus serrani Durio & Manter, 1968 chez Variola albimarginata et Variola louti; Prosorhynchus sp. A chez Epinephelus morrhua; Prosorhynchus sp. immature chez Epinephelus coeruleopunctatus. La nouvelle combinaison Neidhartia longivesicula (Bilqees, Khalil, Khan, Perveen & Muti-ur-Rehman, 2009) (Syn. Prosorhynchus longivesicula) est formée. Cet article et des études moléculaires antérieures indiquent qu’il existe de nombreuses espèces de Prosorhynchinae morphologiquement semblables chez les Serranidae, dont la plupart présentent un haut degré de spécificité à l’hôte.
Key words: Bucephalidae / Neidhartia / Prosorhynchus / Epinephelus / Cephalopholis / Variola / New Caledonia
After January 2014: ISYEB, Institut de Systématique, Évolution, Biodiversité (UMR 7205 CNRS, EPHE, MNHN, UPMC), Muséum National d’Histoire Naturelle, Case postale 51, 55 rue Buffon, 75231 Paris Cedex 05, France
Rodney A. Bray – urn:lsid:zoobank.org:author:C053AFDE-D221-4BE1-9B0A-30307A67D4C0
Jean-Lou Justine – urn:lsid:zoobank.org:author:17643DCB-2C9D-4386-BB94-D2F04966B0E9
© R.A. Bray and J.-L. Justine, published by EDP Sciences, 2013
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bucephalid digeneans are frequently found in fishes of the family Serranidae, in particular in members of the subfamily Epinephelinae [8]. For example, Bray & Justine [5] listed 16 species of Prosorhynchus Odhner, 1905 from serranid fishes: P. atlanticus Manter, 1940, P. bulbosus Kohn, 1961, P. caudovatus Manter, 1940, P. chorinemi Yamaguti, 1952, P. epinepheli Yamaguti, 1939, P. freitasi Nagaty, 1937, P. gonoderus Manter, 1940, P. jupe (Kohn, 1967), P. longisaccatus Durio & Manter, 1968, P. mcintoshi (Velasquez, 1959) (this may belong to Neidhartia), P. ozakii Manter, 1934, P. pacificus Manter, 1940, P. platycephali (Yamaguti, 1934), P. promicropsi Manter, 1940, P. serrani Durio & Manter, 1968, and P. thapari Manter, 1953, and added a further species P. maternus Bray & Justine, 2006. Two were missed, namely P. aguayoi Vigueras, 1955 and P. rarus (Kohn, 1970). Later, Bott & Cribb [2] added a further five species, P. jexi Bott & Cribb, 2009, P. lafii Bott & Cribb, 2009, P. robertsthomsoni Bott & Cribb, 2009, P. conorjonesi Bott & Cribb, 2009 and P. milleri Bott & Cribb, 2009 and recently Bott et al. [3] added yet another five species, all from Plectropomus spp., P. lesteri Bott, Miller & Cribb, 2013, P. wrightae Bott, Miller & Cribb, 2013, P. heronensis Bott, Miller & Cribb, 2013, P. munozae Bott, Miller & Cribb, 2013 and P. plectropomi Bott, Miller & Cribb, 2013, making a total of 29 species. Other genera of bucephalids are also reported in serranids, e.g., Neidhartia Nagaty, 1937 (N. neidharti Nagaty, 1937, N. ghardagae Nagaty, 1937, N. coronata Durio & Manter, 1968, N. epinepheli Bott & Cribb, 2009, N. tyleri Bott, Miller & Cribb, 2013, N. haywardi Bott, Miller & Cribb, 2013, N. plectropomi Bott, Miller & Cribb, 2013), Pseudoprosorhynchus Yamaguti, 1938 (P. hainanensis Shen, 1990), Rhipidocotyle Diesing, 1858 (R. angusticolle Chandler, 1941, R. clavivesiculum Ku & Shen, 1975), Bucephalus Baer, 1827 (B. heterotentaculatus Bravo-Hollis & Lamothe-Argumedo, 1956), Myorhynchus Durio & Manter, 1968 (M. pritchardae Durio & Manter, 1968), Muraenicola Nolan & Cribb, 2010 (syn: Folliculovarium Gu & Shen, 1983 pre-occupied) (M. xishaensis (Gu & Shen, 1983)), Neoprosorhynchus Dayal, 1948 (N. purius Dayal, 1948) and Telorhynchus Crowcroft, 1947 (T. arripidis Crowcroft, 1947).
Most of these species belong to the subfamily Prosorhynchinae Nicoll, 1914, but Bucephalus and Rhipidocotyle are in the Bucephalinae. These may be accidental records. The only bucephaline species originally described from a serranid is R. clavivesiculus which, according to the original description [22], has a recurved pars prostatica and sperm duct, a characteristic of the Prosorhynchinae [33].
This paper expands on the records made in Justine et al. [19], discussing the systematics of the reports in that paper, and adding new data obtained subsequently.
Materials and methods
Digeneans were collected live, immediately fixed in nearly boiling saline and then transferred to 80% ethanol. Whole mounts were stained with Mayer’s paracarmine, cleared in beechwood creosote and mounted in Canada balsam. Measurements were made through a drawing tube on an Olympus BH-2 microscope, using a Digicad Plus digitising tablet and Carl Zeiss KS100 software adapted by Imaging Associates, and are quoted in micrometres. The following abbreviations are used: BMNH, British Museum (Natural History) Collection at the Natural History Museum, London, UK; MNHN JNC, Muséum National d’Histoire Naturelle, Paris, France.
Use has been made of the visual key to Prosorhynchus developed by Bray & Palm [6]. (http://www.nhm.ac.uk/bray2009) and a similar key to the genus Neidhartia recently devised by us. We use the term “cirrus-sac reach” for the distance from the anterior-most extremity of the cirrus-sac to the posterior extremity of the body as a percentage of the body-length.
Results
Family Bucephalidae Poche, 1907
Subfamily Prosorhynchinae Nicoll, 1914
Genus Neidhartia Nagaty, 1937
urn:lsid:zoobank.org:act:380959E0-57F5-44FB-87FE-EB7B4958CCB6
Neidhartia lochepintade n. sp. (Figures 1, 2)
Syn. Prosorhynchus sp. in Epinephelus chlorostigma of Justine et al. (2010).
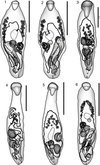 |
Figure 1–6. 1: Neidhartia lochepintade n. sp. Holotype, uterus in outline. 2: Neidhartia lochepintade n. sp. Paratype, uterus in outline. 3: Neidhartia haywardi Bott, Miller & Cribb, 2013, uterus in outline. 4: Neidhartia tyleri Bott, Miller & Cribb, 2013 ex Plectropomus leopardus, uterus in outline. 5: Neidhartia tyleri Bott, Miller & Cribb, 2013, ex Plectropomus laevis, uterus in outline. 6: Prosorhynchus robertsthomsoni Bott & Cribb, 2009. Ventral view, uterus in outline. Scale bars: 500 μm (Figs. 1, 2, 4–6); 200 μm (Fig. 3). |
urn:lsid:zoobank.org:act:A3A03B8A-A686-4168-AFE5-5F6A54C923BA
Type-Host: Epinephelus chlorostigma (Valenciennes) brown-spotted grouper, Serranidae.
Site: Pyloric caeca.
Type-Locality: Off Récif Toombo, deep-sea (22°34′431S, 166°27′552E, 04/01/2008);
Other locality: Off Récif Toombo, deep-sea, 200–300m (22°34′187S, 166°26′292E, 01/12/2009).
Prevalence: 67% (2 of 3).
Type-specimens: Holotype MNHN JNC 2446d-1, Paratypes, MNHN JNC 2446d-2-5, JNC 3141, BMNH 2013.11.18.1.
Etymology: Loche Pintade is the New Caledonian name for the host species.
Description
Based on 10 whole-mount preparations. Measurements and ratios in Table 1. Body fusiform, widest at about mid-body (Figures 1, 2). Tegument spinous; spines squamous, tiny, reach to posterior extremity. Rhynchus broad, relatively short and blunt. Mouth at about level of ovary, distinctly in post-equatorial half of body. Pharynx small, globular. Caecum oval, directed anteriorly.
Measurements and ratios of Neidhartia spp. % refers to % of body-length.
Testes 2, irregularly oval, oblique, in about mid-body, usually well separated. Cirrus-sac elongate, more-or-less parallel sided, reaching anterior testis, anteriorly to pharynx. Seminal vesicle elongate-oval, in proximal cirrus-sac. Pars prostatica long, in two distinct parts; proximal part narrow, coiled over seminal vesicle; distal part wider, straighter, surrounded by dense layer of gland-cells, lining of filaments in chevron arrangement. Ejaculatory duct narrow, opening on large, complex genital lobe inside genital atrium. Genital atrium large. Genital pore distinctly separated from posterior extremity.
Ovary oval, intertesticular, overlapping posterior testis. Mehlis’ gland overlapping ovary and posterior testis. Details of proximal female system obscured by eggs. Uterus not reaching anteriorly to vitelline fields, fills most of available space to level of genital pore. Eggs numerous, tanned, operculate. Metraterm not detected, obscured by eggs. Vitellarium consists of two lateral fields of 12–15 follicles, more or less symmetrical, but with one field slightly longer than other, anterior extremity distinctly posterior to rhynchus and anterior to uterus, always anterior to caecum and gonads; posterior extremity at about level of ovary.
Excretory pore terminal; anterior extent of vesicle obscured by eggs.
Discussion
The features that distinguish N. lochepintade from previously described Neidhartia species are discussed below; comparative metrical data in Table 2.
Comparisons of Neidhartia spp., blue shading shows major distinctions, green shading shows minor distinctions.
Neidhartia coronata Durio & Manter, 1968, based on “six somewhat macerated, extended specimens” from the intestine of a “Serranidae”, “probably Epinephelus”, from off New Caledonia [9], is narrower, with a larger rhynchus, longer previtelline distance, longer pre-uterine distance, longer pre-mouth distance, shorter post-testicular distance, shorter cirrus-sac reach and greater egg-size. The host identifications in Durio & Manter [9] are often rather vague, and this case is no exception. In this particular case, Durio & Manter’s “Epinephelus” could be any Serranidae, including any species of Cephalopholis, Plectropomus, Variola and even Epinephelus.
Neidhartia epinepheli Bott & Cribb, 2009, based on two specimens from the intestine of the highfin grouper Epinephelus maculatus (Bloch) (Serranidae) off Lizard Island on the Great Barrier Reef [2], has a relatively larger rhynchus and a longer previtelline distance. In N. epinepheli the uterus reaches anterior to the vitellarium. Other probably differences are the pre-mouth distance, post-testicular region and cirrus-sac reach.
Neidhartia ghardagae Nagaty, 1937, based on 16 specimens from a “Serranus sp.” from off Ghardaga in the Red Sea [29], has a relative larger rhynchus, longer previtelline distance and longer pre-mouth distance and probably a shorter post-testicular region and a shorter cirrus-sac reach.
Neidhartia haywardi Bott, Miller & Cribb, 2013, based on 10 specimens and ITS2 sequence from Plectropomus leopardus, P. laevis and the spotted coralgrouper P. maculatus (Bloch), from Heron and Lizard Islands on the Great Barrier Reef [3] has a bigger rhynchus, longer previtelline distance and shorter post-testicular distance.
Neidhartia longivesicula (Bilqees, Khalil, Khan, Perveen & Muti-ur-Rehman, 2009) n. comb. (Syn. Prosorhynchus longivesicula) is based on seven specimens from the yellow-tail scad Atule mate (Cuvier) (as Caranx affinus Rüppell) (Carangidae) off Karachi in the northern Arabian Sea [1]. The ovary is described as “posterior to anterior testis and ventro-lateral to posterior testis”, indicating that the species belongs to Neidhartia. This species differs from N. lochepintade particularly in the more anterior mouth and greater body-size.
Neidhartia mcintoshi Velasquez, 1959, based on two mature and four immature specimens from the muscle, stomach and intestine of the duskytail grouper Epinephelus bleekeri (Vaillant) (Serranidae) off Malabon, Rizal, Luzon Island, Philippines [48], has a longer pre-uterine extent, and probably a relatively larger rhynchus, shorter pre-mouth distance and shorter cirrus-sac reach. In connection with unusual sites of infection given, Velasquez [48] stated that the “present species occurs as metacercaria and adult in the same host, showing evidence that infection of one fish is brought about possibly through the eating of the smaller fish by the larger”.
Neidhartia microrhyncha Chauhan, 1943, based on five non-ovigerous specimens from the alimentary canal of the Indian spiny turbot Psettodes erumei (Bloch & Schneider) (Psettodidae) off Bombay (now Mumbai), India [7], is narrower and has a shorter cirrus-sac reach. It is reported to grow much bigger.
Neidhartia neidharti Nagaty, 1937, based on eight specimens from Serranus sp. locally called “Nagil”, from off Ghardaga in the Red Sea [29], has a relatively larger rhynchus and longer pre-mouth distance and probably a shorter post-testicular region and a shorter cirrus-sac reach. The vitellarium overlaps the rhynchus. According to Froese & Pauly [15] the common name “Nagil” refers to either the squaretail coralgrouper Plectropomus areolatus (Rüppell, 1830) or the roving coralgrouper Plectropomus pessuliferus (Fowler, 1904) (Serranidae).
Neidhartia plectropomi Bott, Miller & Cribb, 2013 based on 10 specimens and ITS2 sequence from Plectropomus leopardus and P. laevis from Heron and Lizard Islands on the Great Barrier Reef [3] has a bigger rhynchus and longer previtelline distance.
Neidhartia polydactyli Manter, 1953, based on a single specimen from the intestine of the striped threadfin Polydactylus plebeius (Broussonet) (Polynemidae) off Suva, Fiji [26], has a relatively larger rhynchus and longer previtelline and pre-mouth distances.
Neidhartia tyleri Bott, Miller & Cribb, 2013 based on 10 specimens and ITS2 sequence from the Plectropomus leopardus, P. laevis and P. maculatus, from Heron and Lizard Islands on the Great Barrier Reef [3] is narrower, with longer previtelline and pre-mouth distances, shorter post-testicular distance and cirrus-sac reach, and larger eggs.
Pseudoprosorhynchus hainansis Shen, 1990, based on two specimens from the intestine of the Plectropomus leopardus off Hainan Island, southern China [41] is similar to Neidhartia lochepintade (and indeed the whole genus) in that the ovary is between the testes, but the rhynchus is disc-like, and the worm is long and narrow. It also appears to have a short cirrus-sac reach and smaller eggs.
These data, and the record from this deep-water serranid, indicate to us that the specimens described here belong to a new species. Prosorhynchus epinepheli Yamaguti, 1939 has been reported twice from this host, from off Tuticorin, India [18] and from the Arabian Gulf [40]. The illustrations in both papers show that the ovary lies partly anterior to and partly overlapping the anterior testis, and thus do not indicate that the worm in question is a Neidhartia. The Indian record [18] is from several host species and it is not stated from which the illustrated worm was collected. E. chlorostigma has also been listed as a host for unnamed Prosorhynchus spp. in the Arabian Gulf [11, 39].
As discussed below, the generic status of Prosorhynchus epinepheli and P. longisaccatus is ambiguous as often the ovary does not lie distinctly anteriorly to the testes, suggesting that they may be Neidhartia spp. Comparison of data in Tables 2 and 6 indicates that the rhynchus is relatively much larger in P. epinepheli and P. longisaccatus. The pre-uterine distance tends to be larger in P. longisaccatus, but overlaps considerably.
Neidhartia haywardi Bott, Miller & Cribb, 2013 (Figure 3)
urn:lsid:zoobank.org:act:47F33650-B6E4-414C-9F58-320F4F05E504
Host: Plectropomus leopardus (Lacepède) (Perciformes: Serranidae), leopard coralgrouper.
Site: digestive tract
Localities: Grande Rade, Nouméa 22°15′S 166°24E, 23/10/2007 and 24/10/2007; Between Larégnière and Récif Crouy, 22°20′702S, 166°19′295E, 05/05/2008.
Prevalence: 57% (4 of 7).
Vouchers: MNHN JNC2333B, JNC2333C, JNC2334, JNC2513; BMNH 2013.11.18.5-6.
Description
Based on five whole-mount preparations. Measurements and ratios in Table 1. Body widest at about mid-body (Figure 3). Tegument spinous; spines squamous, tiny, reach to posterior extremity. Rhynchus broad, conical or bluntly conical. Mouth just posterior to ovary, well into post-equatorial half of body. Pharynx small, globular. Caecum oval, directed anteriorly.
Testes 2, irregularly oval, oblique, in about mid-body, usually well separated. Cirrus-sac elongate, more-or-less parallel sided, reaching to or almost to anterior testis, anteriorly to pharynx. Seminal vesicle elongate-oval, in proximal cirrus-sac. Pars prostatica long, in two distinct parts; proximal part narrow, coiled over seminal vesicle; distal part, wider, straighter, surrounded by dense layer of gland-cells, lining of filaments in chevron arrangement. Ejaculatory duct narrow, opening on large, complex genital lobe, inside genital atrium. Genital atrium large. Genital pore distinctly separated from posterior extremity.
Ovary oval, intertesticular, overlapping testes. Mehlis’ gland overlapping ovary and posterior testis. Details of proximal female system obscured by eggs. Uterus reaches anteriorly to vitelline fields, occasionally to level of vitellarium, fills much of available space to level of genital pore. Eggs numerous, tanned, operculate. Metraterm not detected, obscured by eggs. Vitellarium consists of two lateral fields of 12–15 follicles, more or less symmetrical, but with one field slightly longer than other, anterior extremity posterior to rhynchus and anterior extent uterus, reaches anterior to caecum and gonads; posterior extremity at about level of ovary.
Excretory pore terminal; anterior extent of vesicle obscured by eggs.
Discussion
This form appears to be N. haywardi or N. plectropomi differing only in the previtelline distance, as calculated from the illustration [3, Figure 3], but it should be noted that in both species Bott et al. [3] found that the extent of the vitellarium was obscured by the uterus. N. haywardi and N. plectropomi are sister species according to the molecular study of Bott et al. [3]. We consider our specimens to be P. haywardi as the egg-sizes more nearly coincide (Table 2), but the cirrus-sac reach of our specimens tends to be greater than is apparent in either species. Both P. haywardi and P. plectropomi are reported from P. leopardus and P. laevis, and from Heron and Lizard Islands on the Great Barrier Reef.
The features distinguishing this species from its congeners can be seen in Table 2, and two further species are not easily distinguished, namely N. neidharti Nagaty, 1937 and N. epinepheli Bott & Cribb, 2009.
N. neidharti was first reported in Serranus sp. locally known as “Nagil” from the Red Sea [29]. According to Froese & Pauly [15] this common name refers to the squaretail coralgrouper Plectropomus areolatus (Rüppell) or the roving coralgrouper P. pessuliferus (Fowler). It seems clear, therefore, that it is a parasite of Plectropomus. Chauhan [7] recorded, but did not describe, this species in Belone sp. (Beloniformes: Belonidae) from Mumbai (Bombay), India. As unlikely as this combination of hosts is, its putative hosts associations become even more puzzling when the record by Maurya et al. [27] in the freshwater long-whiskered catfish Sperata (= Mystus) aor (Hamilton) (Siluriformes: Bagridae) from Uttar Pradesh, India is considered. We are discounting the Indian records of this species. N. neidharti apparently grows to a much greater size than N. plectropomi, although there is room for confusion. In Nagaty’s [29] description (p. 119) the length range is given as 561–908, whereas in the table of measurements (p. 166) the length is given as 842–2,112 (vs. 658–744 (715) for P. haywardi). This confusion also applies to width where, using the data from the description, the range is 24–27% and in the table it is 11–29% of body-length (vs. 20–24%). The body-width in Nagaty’s Figure 56 is about 24% of the body-length. The pre-mouth distance may be greater than in N. haywardi.
Neidhartia epinepheli. Bott & Cribb stated that it “It bears a superficial resemblance to the type-species, N. neidharti Nagaty, 1937, in that its uterus extends past the posterior margin of the rhynchus. N. epinepheli differs by having a caecum that does not extend into the anterior third of the body and the eggs are smaller, 25–26 × 12–13, compared with 30 × 15 for N. neidharti (see Nagaty, 1937)”. The confusion in the egg-size as given by Nagaty [29] for N. neidharti, in that he gives the egg-size as 30 × 15 in the text, but 19–29 × 15–19 in the table may well invalidate one of Bott & Cribb’s [2] distinctions. The other distinction is rather minor and it may be found that these species are synonymous. The pre-uterine distance is shorter than in N. haywardi in that the uterus overlaps the rhynchus.
Neidhartia tyleri Bott, Miller & Cribb, 2013 (Figures 4, 5)
urn:lsid:zoobank.org:act:E131C73F-7D32-4B80-8656-EB8411FAAE8B
Hosts: Plectropomus leopardus (Lacepède) (Perciformes: Serranidae), leopard coralgrouper; Plectropomus laevis (Lacepède), blacksaddled coralgrouper.
Site: digestive tract
Localities: (P. leopardus & P. laevis) Off Ouano (21°49′430S, 166°44′278E, 25/10/2007), P. leopardus Near Récif Toombo (22°34′107S, 166°28′816E, 30/09/2009).
Prevalences: P. leopardus, 29% (2 of 7), P. laevis, 50% (1 of 2).
Vouchers: (P. leopardus) MNHN JNC2340, JNC 3060B; BMNH 2013.11.18.2-3; (P. laevis) JNC2339; BMNH 2013.11.18.4.
Description
Based on seven whole-mount preparations from P. leopardus and six from P. laevis. Measurements and ratios in Table 1. Body fusiform, widest in posterior third (Figures 4, 5). Tegument spinous; spines squamous, tiny, reach to posterior extremity. Rhynchus broad, with narrow conical posterior extension. Mouth at about level of ovary or just posterior, well into post-equatorial half of body. Pharynx small, globular. Caecum elongate-oval, directed anteriorly.
Testes 2, irregularly oval, oblique to tandem, in about mid-body, slightly separated or not. Cirrus-sac elongate, more-or-less parallel sided, reaching anterior testis, anteriorly to pharynx. Seminal vesicle elongate-oval, in proximal cirrus-sac. Pars prostatica long, in two distinct parts; proximal part narrow, coiled over seminal vesicle; distal part, wider, straighter, surrounded by dense layer of gland-cells, lining of filaments in chevron arrangement. Ejaculatory duct narrow, opening on large, complex genital lobe inside genital atrium. Genital atrium large. Genital pore distinctly separated from posterior extremity.
Ovary oval, intertesticular, overlapping testes. Mehlis’ gland overlapping ovary and posterior testis. Details of proximal female system obscured by eggs. Uterus not reaching anteriorly to vitelline fields, fills much of available space to level of genital pore. Eggs numerous, tanned, operculate. Metraterm not detected, obscured by eggs. Vitellarium consists of two lateral fields of follicles, more or less symmetrical, but with one field slightly longer than other, anterior extremity distinctly posterior to rhynchus and anterior to uterus, always reaches anterior to caecum and gonads; posterior extremity at or just posterior to level of ovary.
Excretory pore terminal; anterior extent of vesicle obscured by eggs.
Discussion
We have identified the larger Neidhartia specimens as belonging to N. tyleri. Most morphological characters are similar (Table 2), but the eggs in our specimens from P. leopardus (the type-host of N. tyleri) are distinctly smaller than those described for this species [3] and our specimens from P. laevis. This species is readily distinguished from most described species (Table 2). N. neidharti is not distinguishable from the specimens from P. laevis in major features of the visual key and differs from the P. leopardus specimens only in rhynchus length (Table 2). This feature probably distinguishes this form from N. neidharti as the P. laevis specimens do not overlap in this feature. Comparison with N. neidharti as described by Nagaty [29] is problematical as the measurements given in the description and table do not coincide, but our specimens are very distinct from the illustrated specimen [28, Figure 56] in shape (relatively more elongate, although the measurements in the table do not bear this out), the previtelline distance and pre-uterine distance.
N. coronata Durio & Manter, 1968, described from “Serranidae, probably Epinephelus sp.” from off New Caledonia [9], differs from our specimens in the visual key in the pre-uterine distance and cirrus-sac reach. It should be borne in mind, however, that Durio & Manter [9] stated that their description was “based on six somewhat macerated, extended specimens”. The previtelline distance may also be a distinguishing feature.
Genus Prosorhynchus Odhner, 1905
urn:lsid:zoobank.org:act:21111289-7672-4028-830D-A37199B68E26
Prosorhynchus robertsthomsoni Bott & Cribb, 2009 (Figure 6)
urn:lsid:zoobank.org:act:0EEE6ED7-01CE-45A0-A2B9-27F32EBC64CC
Host: Cephalopholis argus Bloch & Schneider (Perciformes: Serranidae), peacock hind.
Site: digestive tract
Locality: Near Récif Toombo (22°31′30″S, 166°26′40″E, 03/11/2006).
Prevalence: 50% (1 of 2).
Vouchers: MNHN JNC 2110; BMNH 2013.11.18.25.
Discussion
Measurements and ratios are given in Table 3. This species is known only from Cephalopholis argus, the coral hind Cephalopholis miniata (Forsskål) and the bluespotted hind C. cyanostigma (Valenciennes) from off Heron and Lizard Islands on the Great Barrier Reef [2, 3]. Using the visual key our specimens align with four species, in addition to P. robertsthomsoni. Distinctions are tabulated in Table 4.
Measurements and ratios of Prosorhynchus spp. from Cephalopholis spp. % refers to % of body-length.
Comparisons of Prosorhynchus robertsthomsoni, green shading shows minor distinctions.
Prosorhynchus aguayoi Vigueras, 1955 from the greater soapfish Rypticus saponaceus (Bloch & Schneider) (Serranidae) from off Cuba, Curaçao and Jamaica [30, 31, 50] is a very similar species to P. robertsthomsoni but is probably wider and more fusiform, with a longer post-testicular region. The vitellarium reaches the testes in P. aguayoi and the cirrus-sac does not.
Prosorhynchus jexi (syn: P. epinepheli of Durio &Manter (1968)) from the longfin grouper Epinephelus quoyanus (Valenciennes) (Serranidae) from the Great Barrier Reef [2, 9] differs from P. robertsthomsoni in the more restricted uterus. Bott & Cribb [2] considered that the reach of the uterus anterior to the vitellarium is a distinctive feature of P. robertsthomsoni but our observations indicate that this does not always occur (Figure 6). The cirrus-sac does not reach the testes in P. jexi.
Prosorhynchus serrani Durio & Manter, 1968 (syn: Prosorhynchus crucibulus of Nagaty (1937)) from the yellow-edged lyretail Variola louti (Forsskål) (Serranidae) from the Red Sea and off New Caledonia [9, 29] is very similar to P. robertsthomsoni but apparently has a distinctly different shaped rhynchus, in that it has a distinct narrow elongate posterior extension in contrast to the blunt rounded posterior of the P. robertsthomsoni rhynchus. It may be that the vitellarium reaches slightly more posteriorly in P. serrani in that the follicles extend just posterior to the pharynx, rather than just to the pharynx (see below).
Prosorhynchus tsengi Tsin, 1933 is a parasite of the bartail flathead Platycephalus indicus (Linnaeus) (Platycephalidae) off China [42, 47]. Bray & Palm [6] pointed out that the “original illustration of P. tsengi by Tsin [47, Figure 8] shows a lobed rhynchus, apparently with an aperture, and a straight pars prostatica, indicating that the species may in fact belong to the genus Rhipidocotyle”. In addition the rhynchus and eggs appear slightly smaller than in P. robertsthomsoni.
Prosorhynchus longisaccatus Durio & Manter, 1968 (Figures 7–10)
urn:lsid:zoobank.org:act:FAB66691-C264-4A99-9585-FF7BEDC41316
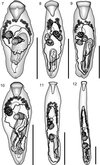 |
Figure 7–12. 7: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Cephalopholis urodeta. Ventral view, uterus in outline. 8: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus areolatus. Ventral view, uterus in outline. 9: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus cyanopodus. Ventral view, uterus in outline. 10: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus maculatus. Ventral view, uterus in outline. 11: Prosorhynchus serrani Durio & Manter, 1968 ex Variola albimarginata. Ventral view, uterus in outline. 12: Prosorhynchus serrani Durio & Manter, 1968 ex Variola louti. Ventral view, uterus in outline. Scale bars: 500 μm. |
Hosts: Cephalopholis urodeta (Forster), Serranidae, darkfin hind; Epinephelus areolatus (Forsskål), Serranidae, areolate grouper; Epinephelus cyanopodus (Richardson), Serranidae, speckled blue grouper; Epinephelus maculatus (Bloch), Serranidae, highfin grouper.
Site: Intestine, pyloric caeca, stomach, digestive tract.
Locality : C. urodeta, Off Récif Kué, New Caledonia (07/10/2008); E. areolatus, Off Pointe Bovis (22°14′S, 166°20′E, 21/10/2008); Nouméa Fish Market (15/06/2007); E. cyanopodus, Passe de Dumbéa (22°20′00″S, 166°15′00″E, 25/11/2005 and 05/10/2006), Passe de Boulari (22°30′00S, 166°24′00″E, 05/10/2006), Near Îlot Mato (22°33′E, 166°47′E, 05/08/2007), Baie Maa (22°12′762S, 166°19′924E, 13/11/2007), Baie des Citrons, Nouméa (22°18′S, 166°26′E, 31/03/2009); E. maculatus, Phare Amédée (22°27′S, 166°26′E, 20/06/2006), Off Ever Prosperity, external slope, depth 60 m; (22°27′S, 166°21′E, 22/08/2006), Off Ever Prosperity, external slope, depth 60–80 m (22°27′S, 166°21′E, 17/04/2007), Récif Kué, External slope (22°34′892S, 166°29′673E, 19/06/2007), Off Récif Kué (22°36′S, 166°31′E, 07/10/2008), Shallow, Interior Lagoon near Récif Toombo (22°32′583S, 166°28′978E, 05/11/2008), Shallow, Interior Lagoon near Récif Toombo (22°32′536S, 166°29′069E, 20/11/2008), Baie des Citrons, Nouméa (22°18′S, 166°26′E, 09/04/2009), Interior Lagoon near Récif Toombo (22°32′536S, 166°29′069E, 30/04/2009).
Prevalence: C. urodeta, 33% (1 of 3); E. areolatus, 75% (3 of 4); E. cyanopodus, 87% (7 of 8); E. maculatus, 61% (16 of 26).
Voucher specimens: C. urodeta, MNHN JNC 2683; E. areolatus, JNC2690, JNC2691, JNC2175; BMNH 2013.11.18.15-16; E. cyanopodus, JNC2270a, JNC1659, JNC1998B, JNC1998C, JNC2000A, JNC2000B, JNC2270, JNC2395, JNC2891A, JNC2891B; BMNH 2006.4.27.1-10, 2013.11.18.22-23; E. maculatus, JNC1874, JNC1927, JNC2157D, JNC2187A, JNC 2680, JNC 2754, JNC 2759, JNC2894B, JNC2929, JNC3031, JNC3052, JNC3053, JNC3061, JNC3066, JNC3067; BMNH 2007.5.2.39-41, 2013.11.18.17-21.
Description
See Tables 3 and 5 for measurements and ratios based on 52 specimens. Ovary in variable position relative to testes: pre-ovarian distance is greater than the pre-testicular distance in the specimen from C. urodeta, in 13 of 16 from E. areolatus, 8 of 13 from E. cyanopodus and 10 of 22 from E. maculatus.
Measurements and ratios of Prosorhynchus longisaccatus from Epinephelus spp. % refers to % of body-length.
Discussion
Our study of this species is based on 52 measured specimens. In our visual key only four species showed no non-overlapping features with our specimens, namely P. atlanticus, P. longisaccatus, P. epinepheli and P. lafii (Table 6). We consider that our specimens conform to the species P. longisaccatus, a species originally reported from a “leche”, a serranid from off New Caledonia [9]. Later, we [5] considered our specimens from E. cyanopodus as this species and then [19] reported E. areolatus, and E. maculatus as hosts; all these reports are from New Caledonia. In the latter paper we reported the specimen from C. urodeta as Prosorhynchus sp.
Comparisons of Prosorhynchus longisaccatus, green shading shows minor distinctions.
Prosorhynchus atlanticus Manter, 1940 is an Atlantic species, originally described in the serranids, the black grouper Mycteroperca bonaci (Poey), the gag Mycteroperca microlepis (Goode & Bean) and the yellowfin grouper Mycteroperca venenosa (Linnaeus) off Florida [25]. The ovary is, apparently, always pre-testicular, the uterus almost never reaches anteriorly to ovary (only slightly in 1 of 29) and the cirrus-sac reach is generally smaller (Table 6).
Prosorhynchus epinepheli Yamaguti, 1939 was originally described from the Hong Kong grouper Epinephelus akaara (Temminck & Schlegel) (Serranidae) from the Inland Sea of Japan [52]. The name has been widely used subsequently for Prosorhynchus specimens from serranids [8], although some may be misidentified. P. longisaccatus is closely similar to P. epinepheli. We believe that either P. epinepheli or P. longisaccatus is the most appropriate identification, particularly as the variable position of the ovary, which is anterior to (and overlapping) the testes or between the testes in our specimens is similar to that described for both of these species. Yamaguti [52] described the position of the ovary in P. epinepheli as “overlapping right testis or entirely on its dorsal side (in the type it lies anterodorsal to the right testis, but may be dorsal, dorsolateral or posterodorsal to it)”. Durio & Manter [9] found that in P. longisaccatus the ovary is “to the right of, or partly posterior to, anterior testis”. This sheds some doubt on the generic classification of the worm, the variation of which includes a characteristic feature of the genus Neidhartia Nagaty, 1937, which according to Overstreet & Curran [33] is “Ovary at level between testes”. Durio & Manter [9] compared their new species to P. epinepheli, using Yamaguti’s [52] original description and new material reported from the honeycomb grouper Epinephelus merra Bloch, 1793 off Heron Island, southern Great Barrier Reef. It should be noted, however, that Bott & Cribb [2] examined one of Durio & Manter’s “P. epinepheli” specimens and considered that it belonged to their new species P. jexi, and that the host was most probably not E. merra, but the similar species, the longfin grouper Epinephelus quoyanus (Valenciennes), which is much commoner in the waters around Heron Island (see also [20]). Durio & Manter [9] summarised the differences between P. epinepheli and P. longisaccatus as “(1) the uterus does not extend even to midatrial level, whereas in all specimens of P. epinepheli it extends postatrially; (2) the rhynchus is wider, and the arrangement of muscles at its anterior edge gives a distinctive appearance”. The first distinction probably relies just on the amount of eggs produced and the second is rather vague and difficult to assess. It seems quite possible that these species are synonymous. There appear to be no morphological criteria for separating these species and we are recognising this species based on the locality of collection, and expect the status of this worm to be elucidated or at least clarified by molecular studies at present in progress.
Prosorhynchus lafii Bott & Cribb, 2009 from the brown-marbled grouper Epinephelus fuscoguttatus (Forsskål) from off Heron Island, Great Barrier Reef [2] differs from P. longisaccatus in the vitelline fields which are “tight lateral clusters at level of caecum”. It is probably a more slender worm than P. longisaccatus (Table 6). The ovary is anterior to, but overlapping, the anterior testis.
Suriano & Martorelli [45] reported P. longisaccatus in the Remo flounder Oncopterus darwinii Steindachner (Pleuronectidae) off Buenos Aires Province, Argentina. It is larger than previously described for this species, with a shorter post-testicular region and cirrus-sac reach, and probably a shorter rhynchus (Table 6). In agreement with Etchegoin et al. [12] we believe that these worms are not conspecific with the worms from serranids in the Pacific Ocean.
Prosorhynchus serrani Durio & Manter, 1968 (Figures 11, 12)
(syn. Prosorhynchus crucibulus (Rudolphi, 1819) from Serranus louti of Nagaty (1937))
urn:lsid:zoobank.org:act:1B73DB12-40AC-419C-9986-EE6EB612A6AF
Hosts: Variola albimarginata Baissac, Serranidae, white-edged lyretail; Variola louti (Forsskål), Serranidae, yellow-edged lyretail.
Site: digestive tract.
Locality: V. albimarginata, Off Ever Prosperity, external slope, depth 60m (22°27′S, 166°21′E, 07/11/2006); V. louti, Near Passe de Dumbéa (22°20′00″S, 166°15′00″E, 01/03/2006, 02/03/2006); Off Ever Prosperity, external slope, depth 60m (22°27′S, 166°21′E, 07/11/2006); Récif Kué, External slope (22°34′892S, 166°29′673E,21/06/2007); External Slope of Récif Toombo (22°33′866S, 166°26′597E, 09/10/2007); Récif Toombo (22°33′172S, 166°26′589E, 20/11/2007).
Prevalence: V. albimarginata, 1 of 1; V. louti, 6 of 10 (60%).
Vouchers: V. albimarginata, MNHN JNC2115; V. louti, JNC1756, JNC1757, JNC2117, JNC2198, JNC2301, JNC2401; BMNH 2007.11.14.44, 2013.11.18.13-14.
Description
It should be noted that the uterus reaches anteriorly beyond the ovary. Measurements and ratios are given in Table 7.
Measurements and ratios of Prosorhynchus serrani. % refers to % of body-length.
Discussion
Prosorhynchus serrani is known previously only from the yellow-edged lyretail Variola louti (Forsskål) (Serranidae) from the Red Sea and off New Caledonia [9, 29]. Our specimens appear indistinguishable from those described by these authors.
This species is very similar to several other species and their relationships will probably only be resolved by molecular means. However, there seem to be minor morphological features which may allow the continued recognition of the Variola parasites as distinct (Table 8). Of those with no distinction in the parameters used in the visual key two can immediately be distinguished by other features.
Comparisons of Prosorhynchus serrani, green shading shows minor distinctions.
P. attenuatus Siddiqi & Cable, 1960 from the Atlantic bumper Chloroscombrus chrysurus (Girard) (Carangidae) off Puerto Rico was described with a “spherical, suckerlike” rhynchus and it certainly looks like a sucker in the illustration. The pars prostatica is described as “tubular” and appears straight in the illustration [43], thus indicating that it may have been placed in the wrong subfamily.
P. caudovatus Manter, 1940 (syn. P. crucibulus of Eckmann (1932)) from serranids in the waters around Africa [4, 10, 13, 14, 46] has distinctive filamented eggs.
Other similar species are:
Prosorhynchus caballeroi Gupta & Ahmad, 1976 known from one specimen from the shrimp scad Alepes djedaba (Forsskål) (as Caranx kalla Cuvier) (Carangidae) in the Bay of Bengal [17] grows larger than P. serrani, with a smaller rhynchus and a longer previtelline distance.
Prosorhynchus conorjonesi Bott & Cribb 2009 from the barramundi cod Cromileptes altivelis (Valenciennes) (Serranidae) on the Great Barrier Reef [2] grows larger than P. serrani, is much narrower, with a more anterior mouth and a shorter cirrus-sac reach.
Prosorhynchus jexi has a more anterior mouth than P. serrani and a longer post-testicular region [2, 9].
Prosorhynchus milleri Bott & Cribb, 2009 based on two specimens from Variola louti from Lizard Island, Great Barrier Reef [2] is very similar to P. serrani and from one of the same host species. It is said to differ from P. serrani in that the latter has “a uterus that extends anterior to the vitelline follicles into the anterior quarter of the body”. Our results complicate things in that the anterior uterine extent varies considerably in our specimens from V. louti. Judging from the illustration of P. milleri in Bott & Cribb [2] the pre-uterine extent is about 51% of body-length and judging from Durio & Manter’s [9] illustration of P. serrani this ratio is about 17%. Durio & Manter [9] considered P. crucibulum from V. louti of Nagaty [29] a synonym of P. serrani and judging from Nagaty’s illustration the pre-uterine distance is about 31% of body-length. This ratio in our worms varies between 26 and 52%, and without a distinct bimodal pattern (26, 29, 30, 32, 33, 38, 39, 40, 45, 49 and 52%). It may well be that there are two forms here, but we do not as yet have enough data to be certain where to draw the line.
Prosorhynchus robertsthomsoni Bott & Cribb, 2009 is very similar to P. serrani but apparently has a distinctly different shaped rhynchus, in that it has a blunt rounded posterior extension in contrast to the distinct narrow elongate posterior extension of the P. serrani rhynchus [2]. It may be that the vitellarium reaches slightly more posterior in P. serrani in that the follicles extend just posterior to the pharynx, rather than just to the pharynx.
Prosorhynchus thapari Manter, 1953 was based on 17 specimens from the spotted coralgrouper Plectropomus maculatus (Bloch) (Serranidae) from off Fiji [26]. We can detect no morphological distinctions from P. serrani and retain the species as separate based on host distinction, and the knowledge that as yet unpublished studies indicate some specificity and cryptic speciation in the genus. Nevertheless, it may well be that this is the oldest valid name for this species.
Prosorhynchus truncatus Verma, 1936 is based on two specimens, one ovigerous and lost and the other without eggs, from the intestine of the river catfish Cephalocassis jatia (Hamilton) (as Arius j.) (Ariidae) off Puri, Bay of Bengal [49]. It has a more posteriorly situated mouth and a shorter cirrus-sac reach.
Prosorhynchus freitasi Nagaty, 1937 (Figures 13, 14)
urn:lsid:zoobank.org:act:D5FF3B1F-10B1-4447-ACB7-C7D544C36AFE
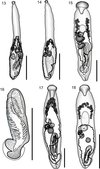 |
Figure 13–18. 13: Prosorhynchus freitasi Nagaty, 1937 from Plectropomus leopardus, uterus in outline. 14: Prosorhynchus freitasi Nagaty, 1937 from Plectropomus laevis, uterus in outline. 15: Prosorhynchus luzonicus Velasquez, 1959, uterus in outline. 16: Prosorhynchus luzonicus Velasquez, 1959, cirrus-sac. 17: Prosorhynchus sp. A ex Epinephelus morrhua. Ventral view, uterus in outline. 18: Prosorhynchus sp. B ex Epinephelus coioides. Ventral view, uterus in outline. Scale bars: 500 μm (Figs. 13–15, 17, 18); 200 μm (Fig. 16). |
Host: Plectropomus laevis (Lacepède), Serranidae, blacksaddled coralgrouper; Plectropomus leopardus (Lacepède), Serranidae, leopard coralgrouper.
Site: digestive tract.
Localities: P. laevis Off Ouano (21°49′430S, 166°44′278E, 25/10/2007); P. leopardus Grande Rade, Nouméa (22°15′S 166°24E, 23/10/2007), Off Ouano (21°49′430S, 166°44′278E, 25/10/2007), Between Larégnière and Récif Crouy (22°20′702S, 166°19′295E, 05/05/2008).
Prevalence: P. laevis 1 of 2 (50%); P. leopardus 5 of 7 (71%).
Vouchers: P. laevis JNC2339; P. leopardus MNHN JNC2333A, JNC 2334, JNC2340, JNC2513, JNC 2514; BMNH 2013.11.18.7-12.
Discussion
Table 9 measurements, Table 10 comparisons.
Measurements and ratios of Prosorhynchus freitasi and P. luzonicus. % refers to % of body-length.
Comparisons of Prosorhynchus freitasi, blue shading shows major distinctions, green shading minor distinctions.
In terms of the parameters used in the visual key there are no differences between our specimens from Plectropomus laevis and Prosorhynchus freitasi as described from “Serranus guttatus” from the Red Sea [29]. According to Froese & Pauly [15] S. guttatus is now known as the peacock hind Cephalopholis argus (Bloch) (Serranidae). It has also been reported in Epinephelus sp. and the spotted coralgrouper Plectropomus maculatus (Bloch) (Serranidae) from off New Caledonia [9] and Plectropomus leopardus and Plectropomus laevis from the Great Barrier Reef [3]. It has an unusual morphology in that all the internal organs are restricted to the posterior half of the body and the rhynchus is relatively small.
Bott et al. [3] described six Prosorhynchus species from Plectropomus spp. on the Great Barrier Reef, five of which are new and one, P. freitasi already known. They are mostly distinguished by minor morphological characters and by analysis of ITS2 rDNA sequences. P. lesteri is distinguished by its distinctly larger rhynchus. P. wrightae differs in the pre-uterine extent, being the only one of these species where the uterus extends well beyond the vitellarium anteriorly. P. heronensis also has a larger rhynchus, although not as large as in P. lesteri, and a distinct U-shaped seminal vesicle. In P. plectropomi the uterus extends to, or just anterior to the anterior extent of the vitellarium, apparently forcing the anterior follicles apart, breaking up the continuous arc found in other related species. P. munozae is a rather small worm, but with larger eggs. Our specimens agree closely with Bott et al.’s [3] description of P. freitasi.
Prosorhynchus luzonicus Velasquez, 1959 (Figures 15, 16)
urn:lsid:zoobank.org:act:25F350A1-F852-4CA9-91D8-A288F9B3F7DD
Host: Epinephelus coioides (Hamilton) (Serranidae), orange-spotted grouper.
Site: Digestive tract.
Locality: Fish Market, Nouméa (14/10/2010).
Prevalence: 1 of 1.
Vouchers: MNHN JNC3277; BMNH 2013.11.18.24.
Discussion
See Table 9 for measurements and Table 11 for morphological comparisons. These specimens from E. coioides are clearly different from those from this host mentioned below as Prosorhynchus sp. B, particularly in pre-mouth distance (see Table 13) and vitelline distribution, but also in post-testicular distance and cirrus-sac reach. On the other hand they are very similar to P. luzonicus as originally described [48] from the barramundi Lates calcarifer (Bloch), (Latidae) from Malabon, Rizal, Luzon island, Philippines. It has been reported in E. coioides in Lampung Bay, southern Sumatra, Indonesia [34–36]. Rückert [35] described and illustrated this species from Epinephelus fuscoguttatus, also from Lampung Bay, and later reported it again in this host, both in culture and in the wild [38]. It is slightly disconcerting that Rückert et al. [37] failed to find this species in L. calcarifer in her study of Lampung Bay. Two useful, but not infallible, recognition features are the separated vitelline fields (occasionally they appear to form an arch), and the mainly postovarian uterus (but according to the figure and illustration by Rückert [35] this is not invariable). Our specimens differ slightly from Velasquez’s [48] description in the greater extent of the cirrus-sac reach as a proportion of body-length (43–51% vs. about 38%). Rückert [35] shows a proportion of about 39%.
Comparisons of Prosorhynchus luzonicus, green shading shows minor distinctions.
Prosorhynchus jexi is similar, but differs in cirrus-sac extent, in the arched vitelline fields and in the extension of the uterus anterior to the ovary (but note that these latter features appear to be variable in P. luzonicus) [2, 9].
Prosorhynchus maternus Bray & Justine, 2006 from the Malabar grouper Epinephelus malabaricus (Bloch & Schneider) off New Caledonia [5] differs in size, post-testicular region and cirrus-sac reach.
Prosorhynchus pacificus Manter, 1940 is an eastern Pacific form, having been reported originally from the serranids, the sailfin grouper Mycteroperca olfax (Jenyns), the broomtail grouper Mycteroperca xenarcha Jordan and an unidentified grouper off the Galapagos [24]. Later records were summarised by Bray & Justine [5], who re-measured two type-specimens. Slight differences from our specimens can be detected in previtelline, pre-mouth and post-testicular distances, cirrus-sac reach and egg-size range. Some specimens from cultured E. coioides in Vietnam have been identified as this species, others as P. epinepheli [51].
Prosorhynchus robertsthomsoni (see above, including new data) differs slightly in pre-uterus, pre-mouth and post-testicular distances [2].
Prosorhynchus squamatus Odhner, 1905 is a Northern Hemisphere species, originally reported from the shorthorn sculpin Myoxocephalus scorpius (Linnaeus) (Cottidae) [32], but since reported in many cold-water hosts [16, 21]. It differs from P. luzonicus in width, previtelline, pre-uterine, pre-mouth and post-testicular distances, cirrus-sac reach and probably in its arched vitellarium and pre-ovarian uterine extent.
Prosorhynchus sp. A (Figure 17)
Epinephelus morrhua (Valenciennes, 1833), Serranidae, comet grouper.
Site: digestive tract.
Locality: Off Récif Kué, deep-sea (22°35′511S, 166°9′893E, 23/01/2008).
Prevalence: 1 of 4 (25%).
Vouchers: MNHN JNC2453; BMNH 2013.11.18.26.
Discussion
No species are identical to these two specimens according to the visual key (Tables 12, 13). As only one specimen is in good condition, the worms have not been described as new, but the very elongate rhynchus seems to be a distinguishing feature. Also note that the ovary lies beside the anterior testis.
Measurements and ratios of Prosorhynchus spp. from Epinephelus spp. % refers to % of body-length.
One species, Prosorhynchus epinepheli, has one major distinguishing feature in the visual key, i.e., width (Table 13). Minor distinguishing features are the pre-mouth distance and the egg-size.
Comparisons of Prosorhynchus spp. from Epinephelus spp., blue shading shows major distinctions, green shading minor distinctions.
Prosorhynchus sp. B. (Figure 18)
Host: Epinephelus coioides (Hamilton, 1822) (Serranidae), orange-spotted grouper.
Site: Digestive tract.
Locality: Fish Market, Nouméa (27/11/2009).
Prevalence: 1 of 1.
Vouchers: MNHN JNC3140; BMNH 2013.11.18.27.
Discussion
Measurements of the three specimens are given in Table 12. Several species are very similar to Prosorhynchus sp. B, and show no differences in the visual key but may be distinguished by combinations of minor features (Table 13). More specimens are needed to describe this form as new as so many similar Prosorhynchus species are known.
Prosorhynchus caudovatus Manter, 1940 (syn. P. crucibulus of Eckmann (1932)) from serranids in the waters around Africa [4, 10, 13, 14, 46] has a distinctive filamented egg. It is also distinctly larger than P. sp. B, has a more anterior mouth and a longer post-testicular region.
Prosorhynchus milleri Bott & Cribb, 2009 based on two specimens from Variola louti from Lizard Island, Great Barrier Reef [2] is longer, narrower, with a smaller rhynchus, a longer previtelline region and a shorter post-testicular region. The vitelline fields reach to the pharynx (vs. distinctly anterior).
Prosorhynchus pacificus belongs to a group of Prosorhynchus spp. with the uterus restricted to the post-ovarian region. In this aspect it differs from P. sp. B. It also differs in previtelline distance, pre-mouth distance, post-testicular region and cirrus-sac reach. The vitelline fields reach the ovary (vs. well anterior to the pharynx).
Prosorhynchus paracrucibulus Velasquez, 1959 based on three non-ovigerous worms (presumably metacercariae) from the scales (!) of the Buru glass perchlet Ambassis buruensis Bleeker (Ambassidae) Manila Bay, Paranaque, Rizal, Luzon Island, Philippines [48]. It is a little wider, with symmetrical testes. The worm is not developed sufficiently enough to recognise, but conceivably it is the metacercaria of a serranid parasite.
Prosorhynchus truncatus Verma, 1936 is based on two specimens, one ovigerous and lost and the other without eggs, from the intestine of the river catfish Cephalocassis jatia (Hamilton) (as Arius j.) (Ariidae) off Puri, Bay of Bengal [49]. It is considerably longer than P. sp. It also differs in previtelline distance, pre-mouth distance, post-testicular region and cirrus-sac reach.
Prosorhynchus specimens from cultured E. coioides in Vietnam have been identified as Prosorhynchus luzonicus and P. epinepheli [51].
Prosorhynchus sp. immature
Host: Epinephelus coeruleopunctatus (Bloch, 1790)
Site: Digestive tract.
Locality: Îlot Lebris, off Ouano (21°50′S, 166°45′E, 25/10/2007).
Prevalence: 1 of 3.
Vouchers: MNHN JNC2338.
Discussion
A single unidentifiable immature specimen was recovered from this host species.
Conclusions
The molecular evidence presented by Bott et al. [3] indicated that there are many distinct, but very similar species of prosorhynchines in serranids, especially Epinephelus and Plectropomus. The morphological similarity of these forms has led to many problems in identification, and some unlikely combinations of hosts in the literature, as for example the quoted hosts for Neidhartia neidharti, which in addition to serranids, includes a belonid and a freshwater siluriform. Recent molecular studies of a wide range of digeneans have indicated that most species exhibit oioxenous or stenoxenous specificity and “that no euryxenous host distribution should be accepted on the basis of morphology only” [28]. Although it is dangerous to identify parasites solely on the basis of their hosts, consideration should be taken of the relatedness of the hosts and the geographical distribution.
Cribb et al. [8] discussed the digenean fauna of epinepheline serranids and found that Prosorhynchus was the commonest parasite, both in the Atlantic/Eastern Pacific region and the Indo-West Pacific Region, and is the only bucephalid genus which has “apparently strongly radiated within the Epinephelinae”. Since that paper [8] our knowledge of epinepheline bucephalids has increased markedly [2, 3, 5] reinforcing that point, but suggesting that Neidhartia has also radiated, at least in the Indo-West Pacific region. The morphological distinctions between Prosorhynchus and Neidhartia are minor, but molecular evidence [3] indicates that these distinctions are reflected by the molecules. Those species of Prosorhynchus with a variable ovary configuration (e.g., P. epinepheli, P. longisaccatus) may invalidate this distinction, or may belong to either monophyletic genus.
Acknowledgments
Julie Mounier, Charles Beaufrère, Anaïs Guillou, Audrey Guérin, Damien Hinsinger, Éric Bureau, Chloé Journo, Violette Justine, Amandine Marie, Aude Sigura, Guilhem Rascalou, Géraldine Colli, Lenaïg Hemery, Pierpaolo Brena, Cyndie Dupoux, Isabelle Mary, Adeline Grugeaud, and Marine Briand, students, participated in the parasitological survey. Claude Chauvet (UNC, Nouméa) caught several fishes. Angelo di Matteo (IRD) provided technical help. Certain fishes were identified from photographs by Ronald Fricke (Staatliches Museum für Naturkunde, Stuttgart, Germany) or by Jack Randall (Bishop Museum, Hawaii). The authors wish to report that there are no competing interests.
References
- Bilqees FM, Khalil B, Khan A, Perveen S, Muti-ur-Rehman. 2009. Description of a new species of genus Prosorhynchus Odhner, 1905 (Trematoda: Bucephalidae: Prosorhynchinae) from the fish Caranx affinis (Rupp.) of Karachi coast. Proceedings of Parasitology, 48, 33–42. [Google Scholar]
- Bott NJ, Cribb TH. 2009. Prosorhynchine trematodes (Digenea: Bucephalidae) from epinephelines (Perciformes: Serranidae) on the Great Barrier Reef, Australia. Systematic Parasitology, 72, 57–69. [CrossRef] [PubMed] [Google Scholar]
- Bott NJ, Miller TL, Cribb TH. 2013. Bucephalidae (Platyhelminthes: Digenea) of Plectropomus (Serranidae: Epinephelinae) in the tropical Pacific. Parasitology Research, 112, 2561–2584. [CrossRef] [PubMed] [Google Scholar]
- Bray RA. 1984. Some helminth parasites of marine fishes and cephalopods of South Africa: Aspidogastrea and the digenean families Bucephalidae, Haplosplanchnidae, Mesometridae and Fellodistomidae. Journal of Natural History, 18, 271–292. [CrossRef] [Google Scholar]
- Bray RA, Justine J-L. 2006. Prosorhynchus maternus sp. n. (Digenea: Bucephalidae) from the Malabar grouper Epinephelus malabaricus (Perciformes: Serranidae) off New Caledonia. Folia Parasitologica, 53, 181–188. [PubMed] [Google Scholar]
- Bray RA, Palm HW. 2009. Bucephalids (Digenea: Bucephalidae) from marine fishes off the south-western coast of Java, Indonesia, including the description of two new species of Rhipidocotyle and comments on the marine fish digenean fauna of Indonesia. Zootaxa, 2223, 1–24. [Google Scholar]
- Chauhan BS. 1943. Trematodes from Indian marine fishes. II. On some trematodes of the gasterostome family Bucephalidae (Braun, 1883) Poche, 1907, with description of four new species. Proceedings of the Indian Academy of Sciences, 17, 97–117. [Google Scholar]
- Cribb TH, Bray RA, Wright T, Pichelin S. 2002. The trematodes of groupers (Serranidae: Epinephelinae): knowledge, nature and evolution. Parasitology, 124, S23–S42. [CrossRef] [PubMed] [Google Scholar]
- Durio WO, Manter HW. 1968. Some digenetic trematodes of marine fishes of New Caledonia. Part I. Bucephalidae, Monorchiidae, and some smaller families. Proceedings of the Helminthological Society of Washington, 35, 143–153. [Google Scholar]
- Eckmann F. 1932. Beiträge zur Kenntnis der Trematodenfamilie Bucephalidae. Zeitschrift für Parasitenkunde, 5, 92–111. [CrossRef] [Google Scholar]
- El-Naffar MKI, Gobashy A, El-Etreby SC, Kardousha MM. 1992. General survey of helminth parasite genera of Arabian Gulf fishes (coasts of United Arab Emirates). Arab Gulf Journal of Scientific Research, 10, 99–110. [Google Scholar]
- Etchegoin JA, Timi JT, Cremonte F, Lanfranchi AL. 2005. Redescription of Prosorhynchus australis Szidat, 1961 (Digenea, Bucephalidae) parasitizing Conger orbignianus Valenciennes, 1842 (Pisces, Congridae) from Argentina. Acta Parasitologica, 50, 102–104. [Google Scholar]
- Fischthal JH. 1980. Some digenetic trematodes of marine fishes from Israel’s Mediterranean coast and their zoogeography, especially those from Red Sea immigrant fishes. Zoologica Scripta, 9, 11–23. [CrossRef] [Google Scholar]
- Fischthal JH, Thomas JD. 1968. Digenetic trematodes of marine fishes from Ghana: Families Acanthocolpidae, Bucephalidae, Didymozoidae. Proceedings of the Helminthological Society of Washington, 35, 237–247. [Google Scholar]
- Froese R, Pauly D. 2013. FishBase. World Wide Web electronic publication. Available on http://www.fishbase.org. [Google Scholar]
- Gibson DI. 1996. Guide to the parasites of fishes of Canada Part IV: Trematoda. Canadian Special Publication of Fisheries and Aquatic Sciences, 124(I-IX), 1–338. [Google Scholar]
- Gupta V, Ahmad J. 1976. Digenetic trematodes of marine fishes. On some new and known digenetic trematodes of the family Bucephalidae Poche, 1907 from marine fishes of Puri, Orissa, India. Anales del Instituto de Biología, Universidad de México, Serie Zoología, 47, 9–18. [Google Scholar]
- Hafeezullah M, Siddiqi AH. 1970. Digenetic trematodes of marine fishes of India. Part I. Bucephalidae and Cryptogonimidae. Indian Journal of Helminthology, 22, 1–22. [Google Scholar]
- Justine J-L, Beveridge I, Boxshall GA, Bray RA, Moravec F, Trilles J-P, Whittington ID. 2010. Parasite biodiversity in coral reef fish: an annotated list of parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda and Nematoda) collected in groupers (Serranidae, Epinephelinae) in New Caledonia. Folia Parasitologica, 57, 237–262. [CrossRef] [PubMed] [Google Scholar]
- Justine J-L, Dupoux C, Cribb TH. 2009. Resolution of the discrepant host-specificity of Pseudorhabdosynochus species (Monogenea, Diplectanidae) from serranid fishes in the tropical Indo-Pacific. Acta Parasitologica, 54, 119–130. [CrossRef] [Google Scholar]
- Køie M. 2000. Metazoan parasites of teleost fishes from Atlantic waters off the Faroe Islands. Ophelia, 52, 25–44. [CrossRef] [Google Scholar]
- Ku C-t, Shen J-w. 1975. Studies on the genus Rhipidocotyle Diesing (Bucephalidae, Trematoda) from some marine fishes of China. Acta Zoologica Sinica, 21, 205–211 (in Chinese). [Google Scholar]
- Madhavi R. 1974. Digenetic trematodes from marine fishes of Waltair Coast, Bay of Bengal. Family Bucephalidae. Rivista di Parassitologia, 35, 189–199. [Google Scholar]
- Manter HW. 1940. Digenetic trematodes of fishes from the Galapagos Islands and the neighboring Pacific. Allan Hancock Pacific Expeditions, 2, 325–497. [Google Scholar]
- Manter HW. 1940. Gasterostomes (Trematoda) of Tortugas, Florida. Papers from the Tortugas Laboratory of the Carnegie Institute of Washington, 33, 1–19. [Google Scholar]
- Manter HW. 1953. Two new species of Prosorhynchinae (Trematoda: Gasterostomata) from the Fiji Islands, in Thapar, G. S., Commemoration Volume, 1953, Dayal J, Singh K, Eds. University of Lucknow: Lucknow. p. 193–200. [Google Scholar]
- Maurya AK, Agarwal GP, Singh SPN. 1993. Studies on some known and unknown eucephalid [sic] trematodes from the fresh water fishes. Journal of Scientific Research of the Banaras Hindu University, 43, 61–72. [Google Scholar]
- Miller TL, Bray RA, Cribb TH. 2011. Taxonomic approaches to and interpretation of host specificity of trematodes of fishes: lessons from the Great Barrier Reef. Parasitology, 138, 1710–1722. [CrossRef] [PubMed] [Google Scholar]
- Nagaty HF. 1937. Trematodes of fishes from the Red Sea. Part 1. Studies on the family Bucephalidae Poche, 1907. Cairo: Egyptian University. [Google Scholar]
- Nahhas FM, Cable RM. 1964. Digenetic and aspidogastrid trematodes from marine fishes of Curaçao and Jamaica. Tulane Studies in Zoology, 11, 169–228. [Google Scholar]
- Nahhas FM, Carlson K. 1994. Digenetic trematodes of marine fishes of Jamaica, West Indies. Publications of the Hofstra University Marine Laboratory, Ecological Survey of Jamaica, 2, 1–60. [Google Scholar]
- Odhner T. 1905. Die Trematoden des arktischen Gebietes. Fauna Arctica, 4, 289–374. [Google Scholar]
- Overstreet RM, Curran SS. 2002. Superfamily Bucephaloidea Poche, 1907, in Keys to the Trematoda, Volume 1, Gibson DI, Jones A, Bray RA, (Eds.) CABI Publishing and the Natural History Museum: Wallingford, p. 67–110. [Google Scholar]
- Palm HW, Rückert S. 2009. A new approach to visualize ecosystem health by using parasites. Parasitology Research, 105, 539–553. [CrossRef] [PubMed] [Google Scholar]
- Rückert S. 2006. Marine Fischparasiten in Indonesien: Befallssituation und Bedeutung für die Marikultur von Zackenbarschen, in Mathematisch-Naturwissenschaftlichen Fakultät. Heinrich-Heine-Universität Düsseldorf: Düsseldorf. p. 240. [Google Scholar]
- Rückert S, Klimpel S, Al-Quraishy S, Mehlhorn H, Palm HW. 2009. Transmission of fish parasites into grouper mariculture (Serranidae: Epinephelus coioides (Hamilton, 1822)) in Lampung Bay, Indonesia. Parasitology Research, 104, 523–532. [CrossRef] [PubMed] [Google Scholar]
- Rückert S, Klimpel S, Palm HW. 2008. Parasite fauna of seabass (Lates calcarifer) under mariculture conditions in Lampung Bay, Indonesia. Journal of Applied Ichthyology, 24, 321–327. [CrossRef] [Google Scholar]
- Rückert S, Klimpel S, Palm HW. 2010. Parasites of cultured and wild brown-marbled grouper Epinephelus fuscoguttatus (Forsskal, 1775) in Lampung Bay, Indonesia. Aquaculture Research, 41, 1158–1169. [Google Scholar]
- Saoud MFA, Ramadan MM, Al Kawari KSR. 1986. Helminth parasites of fishes from the Arabian Gulf. 1. Preliminary general survey of fishes mainly from Qatari waters. Qatar University Science Bulletin, 6, 199–229. [Google Scholar]
- Saoud MFA, Ramadan MM, Al Kawari KSR. 1989. Helminth parasites of fishes from the Arabian Gulf. 6. On three species of digenetic trematodes: Prosorhynchus epinepheli Yamaguti, 1939; Paraproctotrema qatarensis n. sp. and Prosorchis breviformis Srivastava, 1936. Rivista di Parassitologia, 49, 79–85. [Google Scholar]
- Shen J-w. 1990. Digenetic trematodes of marine fishes from Hainan Island. Science Publications: Beijing (in Chinese, English summary). [Google Scholar]
- Shen J-w, Qiu Z-z. 1995. Studies on the trematodes of fishes from the Yellow Sea and the Bo Hai Sea. Science Press: Beijing (in Chinese). [Google Scholar]
- Siddiqi AH, Cable RM. 1960. Digenetic trematodes of marine fishes of Puerto Rico. Scientific Survey of Porto Rico and the Virgin Islands, 17, 257–369. [Google Scholar]
- Srivastava HD. 1938. Studies on the gasterostomatous parasites of Indian food fishes. Indian Journal of Veterinary Science and Animal Husbandry, 8, 317–339. [Google Scholar]
- Suriano DM, Martorelli SR. 1983. Estudios parasitologicos en la albufera de Mar Chiquita, provincia de Buenos Aires, Republica Argentina. 1. Steringotrema microacetabularis sp. nov., Prosorhynchus longisaccatus Durio & Manter, 1968 y Lobatostoma ringens (Linton) Eckmann, 1932 (Trematoda) parasites de peces Pleuronectiformes. Neotropica, 29, 195–207. [Google Scholar]
- Szuks H. 1981. Bucephaliden (Trematoda: Digenea) aus Fischen der Küstengewässer Nordwestafrikas. Wissenschaftliche Zeitschrift der Pädagogischen Hochschule “Liselotte Herrmann” Güstrow Aus der Mathematisch-Naturwissenschaftlichen Fakultät, 2, 167–178. [Google Scholar]
- Tsin SN. 1933. Parasitic trematodes in fishes of China. Journal of Science, 1, 379–392 (in Chinese). [Google Scholar]
- Velasquez CC. 1959. Studies on the family Bucephalidae Poche 1907 (Trematoda) from Philippine food fishes. Journal of Parasitology, 45, 135–147. [CrossRef] [Google Scholar]
- Verma SC. 1936. Studies on the family Bucephalidae (Gasterostomata), Part II. Descriptions of two new forms from Indian marine fishes. Proceedings of the National Academy of Sciences, India, 6, 252–260. [Google Scholar]
- Vigueras IP. 1955. Contribucion al conocimiento de la fauna helmintologica cubana. Memorias de la Sociedad Cubana de Historia Natural, 22, 21–71. [Google Scholar]
- Vo DT, Bristow GA, Nguyen DH, Vo DT, Nguyen TNN, Tran TC. 2011. Digenean trematodes of cultured grouper (Epinephelus coioides and E. bleekeri) in Khanh Hoa Province, Vietnam, in Diseases in Asian Aquaculture VII. Fish Health Section, Bondad-Reantaso MG et al., Eds. Selangor, Malaysia: Asian Fisheries Society, p. 39–52. [Google Scholar]
- Yamaguti S. 1939. Studies on the helminth fauna of Japan. Part 26. Trematodes of fishes, VI. Japanese Journal of Zoology, 8, 211–230. [Google Scholar]
Cite this article as: Bray RA & Justine J-L: Bucephalidae (Digenea) from epinephelines (Serranidae: Perciformes) from the waters off New Caledonia, including Neidhartia lochepintade n. sp. Parasite, 2013, 20, 56.
All Tables
Comparisons of Neidhartia spp., blue shading shows major distinctions, green shading shows minor distinctions.
Measurements and ratios of Prosorhynchus spp. from Cephalopholis spp. % refers to % of body-length.
Comparisons of Prosorhynchus robertsthomsoni, green shading shows minor distinctions.
Measurements and ratios of Prosorhynchus longisaccatus from Epinephelus spp. % refers to % of body-length.
Comparisons of Prosorhynchus longisaccatus, green shading shows minor distinctions.
Measurements and ratios of Prosorhynchus freitasi and P. luzonicus. % refers to % of body-length.
Comparisons of Prosorhynchus freitasi, blue shading shows major distinctions, green shading minor distinctions.
Comparisons of Prosorhynchus luzonicus, green shading shows minor distinctions.
Measurements and ratios of Prosorhynchus spp. from Epinephelus spp. % refers to % of body-length.
Comparisons of Prosorhynchus spp. from Epinephelus spp., blue shading shows major distinctions, green shading minor distinctions.
All Figures
 |
Figure 1–6. 1: Neidhartia lochepintade n. sp. Holotype, uterus in outline. 2: Neidhartia lochepintade n. sp. Paratype, uterus in outline. 3: Neidhartia haywardi Bott, Miller & Cribb, 2013, uterus in outline. 4: Neidhartia tyleri Bott, Miller & Cribb, 2013 ex Plectropomus leopardus, uterus in outline. 5: Neidhartia tyleri Bott, Miller & Cribb, 2013, ex Plectropomus laevis, uterus in outline. 6: Prosorhynchus robertsthomsoni Bott & Cribb, 2009. Ventral view, uterus in outline. Scale bars: 500 μm (Figs. 1, 2, 4–6); 200 μm (Fig. 3). |
| In the text | |
 |
Figure 7–12. 7: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Cephalopholis urodeta. Ventral view, uterus in outline. 8: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus areolatus. Ventral view, uterus in outline. 9: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus cyanopodus. Ventral view, uterus in outline. 10: Prosorhynchus longisaccatus Durio & Manter, 1968 ex Epinephelus maculatus. Ventral view, uterus in outline. 11: Prosorhynchus serrani Durio & Manter, 1968 ex Variola albimarginata. Ventral view, uterus in outline. 12: Prosorhynchus serrani Durio & Manter, 1968 ex Variola louti. Ventral view, uterus in outline. Scale bars: 500 μm. |
| In the text | |
 |
Figure 13–18. 13: Prosorhynchus freitasi Nagaty, 1937 from Plectropomus leopardus, uterus in outline. 14: Prosorhynchus freitasi Nagaty, 1937 from Plectropomus laevis, uterus in outline. 15: Prosorhynchus luzonicus Velasquez, 1959, uterus in outline. 16: Prosorhynchus luzonicus Velasquez, 1959, cirrus-sac. 17: Prosorhynchus sp. A ex Epinephelus morrhua. Ventral view, uterus in outline. 18: Prosorhynchus sp. B ex Epinephelus coioides. Ventral view, uterus in outline. Scale bars: 500 μm (Figs. 13–15, 17, 18); 200 μm (Fig. 16). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


