| Issue |
Parasite
Volume 20, 2013
|
|
|---|---|---|
| Article Number | 46 | |
| Number of page(s) | 6 | |
| DOI | https://doi.org/10.1051/parasite/2013046 | |
| Published online | 21 November 2013 | |
Research Article
Efficacy of a topical application of Certifect® (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v) for the treatment of canine generalized demodicosis
Efficacité d’un traitement topique au Certifect® (fipronil 6,26 % w/v, amitraz 7,48 % w/v, (S)-méthoprène 5,63 % w/v) dans le traitement de la démodécie généralisée du chien
1
Merial S.A.S., 29 avenue Tony Garnier, 69630
Lyon, France
2
Clinvet International (Pty) Ltd, PO Box 11186, 9321 Universitas, South Africa
* Corresponding author: frederic.beugnet@merial.com
Received:
5
July
2013
Accepted:
7
November
2013
The efficacy of the treatment with Certifect ® (containing fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v) applied topically was assessed in 18 dogs diagnosed with clinical generalized demodicosis. Three treatment regimens were compared over a 3-month period. Starting at Day 0, dogs were treated monthly (group 1) or every two weeks (group 2) with the combination of fipronil, amitraz, and (S)-methoprene or with monthly topical applications of the combination of amitraz and metaflumizone (group 3, reference treatment). Clinical examinations including deep skin scrapings were performed every month in order to evaluate the resolution of clinical signs and the reduction in mite counts. On Day 84, the percentage reduction of mite counts in group 1 was 99.8%, whereas no Demodex canis could be detected in groups 2 and 3 (i.e. 100% parasitological efficacy). As a result of the Demodex mite count reduction, the skin condition of the dogs improved significantly in all groups. This study illustrates, that both monthly and bi-weekly treatments with Certifect were effective in treating dogs with generalized demodicosis over a 3-month period.
Résumé
L’efficacité du traitement topique avec Certifect® (combinaison de fipronil 6,26 % w/v, amitraz 7,48 % w/v, et (S)-methoprene 5,63 % w/v) a été évaluée chez 18 chiens atteints de démodécie généralisée et porteurs de Demodex canis. Trois protocoles de traitement ont été comparés sur une période de 3 mois. À partir du jour 0, les chiens du groupe 1 ont été traités 3 fois à un mois d’intervalle avec la combinaison d’amitraz, de fipronil et de (S)-méthoprène; les chiens du groupe 2 ont été traités avec la même combinaison administrée à des intervalles de 2 semaines et les chiens du groupe 3 ont été traités par application topique mensuelle d’une combinaison commerciale d’amitraz et de métaflumizone ayant l’indication pour le traitement de la démodécie canine. L’efficacité des différents protocoles a été évaluée au cours d’examens cliniques mensuels accompagnés de raclages cutanés profonds. Au jour 84, le pourcentage de réduction parasitaire dans le groupe 1 était de 99.8 %, il était de 100 % dans les groupes 2 et 3. Les lésions dermatologiques étaient significativement réduites dans les 3 groupes. Cette étude confirme que l’application topique de Certifect pendant 3 mois à un intervalle de 2 semaines ou 1 mois permet de traiter avec succès les chiens atteints de démodécie généralisée.
Key words: Demodex canis / demodicosis / treatment / fipronil/amitraz/(S)-methoprene / dog
© J. Fourie et al., published by EDP Sciences, 2013
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Demodicosis is a parasitic inflammatory skin disease of dogs caused by an excessive proliferation of Demodex canis Leydig, 1859 [8]. A small number of mites may constitute a normal component of the dog’s skin fauna [15,16], but a proliferation of mites can lead to serious disease. The parasite is not considered contagious except during a few days after birth, when puppies acquire mites through direct skin contact from their mother. Three morphologically different types of Demodex have been described and named as species by some authors (D. canis, D. injai, and D. cornei) but a final consensus on taxonomy will require molecular testing [5,9,15]. Published data indicate similar efficacy of reported treatments regardless of the Demodex type [9].
Canine demodicosis can be divided into two clinical manifestations. The localized form appears as small patches of alopecia and mild erythema in young dogs. It generally regresses spontaneously without treatment. The generalized form of demodicosis is more severe and can even be fatal. It may develop from the localized condition or occur in older animals, especially those undergoing severe stress or with underlying diseases. The definition of localized versus generalized demodicosis has been a matter of debate. A recent committee of experts considered demodicosis as localized if there are no more than four lesions with a diameter of up to 2.5 cm in width [5, 9]. Canine generalized demodicosis is frequently seen in practice [13] and is characterized by five or more affected areas or by lesions covering an entire region of the body, and/or pododemodicosis involving two or more paws [3]. The affected areas are erythematous, with comedones, hair loss, follicular papules to pustules and scales. Lymphadenopathy is commonly associated with the disease and secondary bacterial infections are very frequent [5]. Although some young dogs with an early generalized form can self-cure naturally, it is impossible to clinically ascertain which animals will progress to the more severe state. The diagnosis is typically based on clinical signs and is confirmed by the presence of mites in deep skin scrapings. Although Demodex mites are part of the normal microfauna, it is uncommon to find the mites, even by performing several deep skin scrapings. If a mite is found, this should raise suspicion and additional skin scrapings should be performed. Finding more than one mite is strongly suggestive of clinical demodicosis [5, 9].
Despite recent advances with new acaricidal drugs, generalized demodicosis remains a very challenging disease to treat effectively. Amitraz, as a rinse or sponge -on, has been approved for the treatment of canine generalized demodicosis in many countries for decades. Several amitraz-based protocols have been described at various concentrations and frequencies. Efficacy data are reported to be variable, it is time-consuming, and there may be safety issues [11]. Protocols based on daily to weekly oral or subcutaneous injections of macrocyclic lactones including ivermectin, doramectin, and moxidectin have been published but represent off-label use with potential for toxicity, especially in dogs mutated for MDR-1 (P-glycoprotein deficiency), especially including colley breeds [9, 11]. Daily oral milbemycin oxime at a dose of 500 mg/kg is registered in many countries for the treatment of canine demodicosis [7].
The most recent registered treatments for demodicosis include topical products that help improve owner compliance, and therefore increase the rate of success. These drugs, applied as a spot-on at monthly or bi-weekly intervals, contain well-known active compounds like amitraz (combined with the insecticide metaflumizone) or moxidectin (combined with the insecticide imidacloprid) [2, 3, 6, 10]. These new alternatives provide a safer and more convenient approach to cure this disease. Whatever the choice of the antiparasitic drug, the duration of the treatment of demodicosis usually requires 3 months or more.
Certifect® is a spot-on formulation that combines fipronil, amitraz, and (S)-methoprene. The addition of amitraz to fipronil has been shown to significantly potentiate the acaricidal effects of fipronil [12, 14]. The product has been recently demonstrated to be active against sarcoptic mange [4]. Based on these findings, the purpose of the present study was to assess the efficacy of the topical administration of Certifect, against Demodex canis on dogs.
Material and methods
The study was a single center trial including three parallel groups. The study design and the study conditions of this study were approved by the local animal welfare ethic committee in accordance with Good Clinical Practice by the European Agency for the Evaluation of Medicinal Products (CVMP/VICH GL9, July 2000; CVMP/VICH GL19, July 2001). All dogs belonged to private owners who signed an owner consent for the inclusion of their dogs in the study. Dogs were allocated to treatment groups randomly, and all evaluations of efficacy were performed by blinded personnel.
Eighteen mongrel dogs (12 males, 6 females) weighing from 6 to 19 kg and being at least 1-year old were included. All had clinical signs of Demodex infestation such as erythema, hair loss, seborrhea, follicular casts, scales, and crust in at least five spots or on an entire body region. Deep skin scrapings performed at the time of inclusion confirmed an average count of 527 D. canis mites for five lesion sites per dog. Morphological observations of the mites clearly identified them as Demodex canis type. Except for clinical signs of demodicosis, dogs were otherwise declared in good general condition by a veterinarian at the time of enrolment. The health condition of the dogs was monitored daily from inclusion (Day -7) to study end (Day 84). Significant secondary bacterial infections were reasons for exclusion.
Dogs were housed in individual cages preventing contact between animals and were exposed to ambient temperature and natural sunlight. Dogs had access to an individual outside run partly covered with a roof and were not exposed to rain. Food was provided in an amount and manner to maintain normal condition. Water was available ad libitum. Dogs were acclimatized to the study conditions for 7 days before the first treatment.
Experimental design
-
Dogs were randomized into three treatment groups based on decreasing mite counts at inclusion. Six dogs in treatment groups 1 were treated every four weeks with a topical formulation of Certifect (containing fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v per complete volume of the dual cavity pipette) following the label recommendations (Table 1),
Table 1.Dosage recommendation for Certifect® and Promeris Duo®.
-
Six dogs in treatment group 2 were treated every two weeks with Certifect following the label-recommended dosage (Table 1),
-
Six dogs in treatment group 3 were treated every four weeks with a topical formulation of Promeris Duo™ 2 (amitraz 15% w/v, metaflumizone 15% w/v) at the label-recommended dosage (Table 1).
Based on dog weight ranges, the maximum amitraz dosage for Promeris Duo is 50 mg/kg whereas it is 16 mg/kg for Certifect; the minimum dosages are 20 mg/kg for Promeris Duo and 8 mg/kg for Certifect.
Dogs in groups 1 and 3 were treated on days 0, 28, and 56 and dogs in group 2 on days 0, 14, 28, 42, 56, and 70.
Mite counts
Demodex counts were performed on dogs prior to enrolment and on Days 27, 55, and 84. Each count consisted of a deep skin scraping from five lesion sites on each animal. Skin scraping sites were recorded (Table 2) and these sites were scraped at each subsequent examination. The surface of each skin scraping was 4 cm2 and they were made with a blade until capillary oozing occurred which took on average 3 min per site. The scrapings were mixed with oil and examined under a stereomicroscope. The number of live mites (adults and immatures) was counted. The methodology for mite count and clinical evaluation was similar to published efficacy data with other spot-ons in order to allow for comparison [2, 3].
Localization of the five lesion sites retained for scraping sites on each dog.
Clinical evaluation
Clinical signs and the extent of demodectic lesions on each dog were assessed on the days when scrapings were made [2, 3]. Dogs were examined for the presence/absence of scales and crusts and the percentage of body area with hair regrowth. A semi-quantitative assessment of hair regrowth was made. The skin surface of the dogs on which hair regrowth occurred, compared to pre-treatment observations, was scored as 1 if < 50% hair regrowth occurred, 2 if 50–90% hair regrowth occurred, and 3 if > 90% hair regrowth took place.
Data analysis
The primary efficacy variable was the decrease in live mite counts (adults and immatures) following treatment. The average percentage reduction in mite counts for the group was calculated by: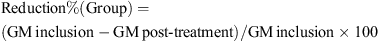 where GM Inclusion = the geometric mean (GM) of the Inclusion (pre-treatment) mite counts, and GM Post-treatment = the geometric mean of the Post-treatment mite counts.
where GM Inclusion = the geometric mean (GM) of the Inclusion (pre-treatment) mite counts, and GM Post-treatment = the geometric mean of the Post-treatment mite counts.
The Inclusion and Post-treatment mite counts within each group were compared using an ANOVA with time (pre- or post-treatment) and dog effects. A 5% level of significance for the within-group comparison (pre-treatment live mite counts vs post-treatment live mite counts) was used. The three groups were compared descriptively with respect to the percent reduction in mite counts. The success rate was defined as the percentage of dogs in each group that were negative for live mites at the time of scraping.
The secondary variable was the resolution of clinical signs. The number of dogs in each group affected by scales and crusts was tabulated at inclusion and at the different post-treatment assessment days. In addition, the number of dogs in each category of hair regrowth was tabulated by group for each post-treatment evaluation.
Results
Antiparasitic efficacy
All the dogs were positive for the presence of live mites prior to the first treatment. For all three treated groups on all post-treatment assessment days, the mite counts were statistically significantly reduced (p < 0.05) compared to the counts at inclusion (Table 3). At the first post-treatment evaluation (Day 27), the percentage reduction in the three groups ranged from 97.4 to 97.9%, with two dogs with no mites identified in groups 1 and 2 each.
Individual Demodex counts for each dog at each evaluation and mean percentage of mite count reduction for each group.
On Day 55, most of the dogs did not harbor Demodex mites and the percentage reductions were 98.5, 99.8, and 99.9% in groups 1, 2, and 3, respectively.
On Day 84, none of the dogs in groups 2 and 3 harbored D. canis, resulting in 100% efficacy. The percentage reduction in group 1 was 99.8% as two dogs still had two mites each.
The difference in mite counts between the three groups was not statistically significant at any time point. Three, four, and five dogs in treatment groups 1, 2, and 3, respectively, had two consecutive negative scrapings at the one-month interval which is considered a cure by dermatologists.
Clinical efficacy
Clinical signs of demodicosis were markedly reduced following the three treatment regimens and correlated with the mite count reductions (Table 2). Scales and crusts completely disappeared in 5/5 dogs in group 1, 3/6 dogs in group 2, and 5/6 dogs in group 3.
The sizes of the affected areas were considerably reduced as shown by hair regrowth (Table 4). On Day 84, all dogs in groups 1 and 2 had a body area with hair regrowth over 90% compared to that recorded during inclusion.
Evaluation of the clinical signs associated with demodicosis and hair regrowth during the 3-month study period.
Health observations
No adverse events related to treatments were observed. One dog (4C5 C66) in study Group 1 was found dead in its cage on Day 12 and the cause was determined from post mortem examinations as being the consequences of demodicosis with secondary bacterial infection and septicaemia.
Discussion and conclusion
The present study demonstrates that treatment with a spot-on formulation of fipronil, amitraz, and (S)-methoprene (Certifect) for 3 months at bi-weekly or monthly intervals resulted in a rapid reduction in mite numbers and a marked improvement in clinical signs in all dogs. A significant portion of group 1 (3/5, 66.7%) and group 2 (6/6, 100%) had no mites in their skin scrapings at the end of the study. The sensitivity of skin scrapings to detect remission of demodicosis has sometimes been challenged. As the life cycle of the mite extends over a period of 18–24 days [16], and considering that scrapings are performed on a limited area of the lesions, a single negative skin scraping should generally not be considered as an indication of complete remission. Remission should rather be determined based on two consecutive negative skin scrapings done at a one-month interval [5, 9]. In the present study, the majority of dogs had two negative skin scrapings, indicating that treatments at appropriate intervals can provide remission of disease. Unfortunately, the duration of the study did not allow a second evaluation of the three dogs that had a few mites at Day 55 and no mites at Day 84. The clear clinical improvement seen on all dogs is another sign of effective treatment. It is known that even without mites, the lesions will disappear slowly in some dogs due to the time needed for skin to fully recover.
Both monthly and bi-weekly treatments with Certifect were regarded as effective in treating dogs with generalized demodicosis. Bi-weekly treatments did however result in a complete disappearance of mites in the scrapings at Day 84.
This study demonstrates that both monthly and bi-weekly applications of Certifect over a period of 70 days are effective in treating dogs with generalized demodicosis. Demodex mite infestations were quickly reduced, resulting in a marked clinical improvement. These results are similar to other published data assessing the efficacy of a metaflumizone-amitraz combination [3]. In this study, the effectiveness of Certifect treatments was comparable to that of Promeris Duo. The Certifect treatment applies an amitraz concentration on dogs that is in average 40% of the Promeris Duo treatment. The comparable effect is probably due to a potentiation with fipronil.
Competing interests
This clinical study was funded by Merial S.A.S., 29 avenue Tony Garnier, 69007 Lyon of which Frederic Beugnet, Lénaïg Halos and Matthias Pollmeier are employees. ClinVet, of which J.J. Fourie is an employee, is an independent, South African, Contract Research Organisation contracted to conduct the study. All authors voluntarily publish this article and have no personal interest in these studies other than publishing the scientific findings that they have been involved in via planning, initiating, monitoring, and conducting the investigations and analyzing the results.
Acknowledgments
The authors would like to thank Catherine Ollagnier who was the Merial R&D monitor at the time of the study.
Certifect ® is a registered trademark of Merial. All other marks are the property of their respective owners. This document is provided for scientific purposes only. Any reference to a brand or a trademark herein is for informational purposes only and is not intended for a commercial purpose or to dilute the rights of the respective owner(s) of the brand(s) or trademark(s).
References
- Folz SD, Kakuk TJ, Hencke CL, Rector DL, Tesar FB. 1984. Clinical evaluation of amitraz as a treatment for canine demodecosis. Veterinay Parasitology, 16, 335–341. [Google Scholar]
- Fourie JJ, Heine J, Horak IG. 2006. The efficacy of an imidacloprid/moxidectin combination against naturally acquired Sarcoptes scabiei infestations on dogs. Australian Veterinary Journal, 84, 17–21. [CrossRef] [PubMed] [Google Scholar]
- Fourie L, Kok D, du Plessis A, Rugg D. 2007. Efficacy of a novel formulation of metaflumizone plus amitraz for the treatment of demodectic mange in dogs. Veterinary Parasitology, 150, 268–274. [CrossRef] [PubMed] [Google Scholar]
- Gaxiola S, Gaxiola J, Perez A, Yoon S, Irwin J, Halos L, Beugnet F, Pollmeier M, Alva R. 2013. Effectiveness of two topical treatments with a combination fipronil/amitraz/(S)-methoprene against natural infestations of mites (Sarcoptes scabiei var. canis) on dogs. International Journal of Applied Research in Veterinary Medecine, 11, 10–15. [Google Scholar]
- Guaguère E, Beugnet F. 2008. Parasitic skin conditions, in A practical Guide to Canine Dermatology, Guaguère E, Prélaud P, Craig M, Eds. Kalianxis: Paris. pp. 179–226. [Google Scholar]
- Heine J, Krieger K, Dumont P, Hellmann K. 2005. Evaluation of the efficacy and safety of imidacloprid 10% plus moxidectin 2.5% spot–on in the treatment of generalized demodicosis in dogs: results of a European field study. Parasitology Research, 97 (Suppl. 1) S89–S96. [CrossRef] [PubMed] [Google Scholar]
- Holm BR. 2003. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: a retrospective study of 99 dogs (1995–2000). Veterinary Dermatology, 14, 189–195. [CrossRef] [PubMed] [Google Scholar]
- Leydig F. 1859. Über Haarsackmilben und Krätzmilben. Archiv für Naturgeschichte, Jahrg. XXV, Bd. I., 338–354 + Table XIII. [Google Scholar]
- Mueller R. 2012. An update on the therapy of canine demodicosis. The Compendium on Continuing Education for Veterinarians, E1–E4. [Google Scholar]
- Mueller R, Meyer D, Bensignor E, Sauter-Louis C. 2009. Treatment of canine generalized demodecosis with a “spot-on” formulation containing 10% moxidectine and 2.5% imidacloprid (Advocate, Bayer Healthcare). Veterinary Dermatology, 20, 441–446. [CrossRef] [PubMed] [Google Scholar]
- Paterson T, Halliwell R, Fields P, Lanza Louw M, Louw J, Ball G, Pinckney R, McKibben J. 2009. Treatment of canine-generalized demodecosis: a blind, randomized clinical trial comparing the efficacy of Advocate (Bayer Animal Health) with ivermectin. Veterinary Dermatology, 20, 447–455. [CrossRef] [PubMed] [Google Scholar]
- Pfister K. 2011. Fipronil, amitraz and (S)-methoprene – a novel ectoparasiticide combination for dogs. Veterinary Parasitology, 179, 293–356. [CrossRef] [PubMed] [Google Scholar]
- Plant J, Lund E, Yang M. 2011. A case control study of the risk factors for juvenile-onset generalized demodecosis in the USA. Veterinary Dermatology, 22, 95–99. [CrossRef] [PubMed] [Google Scholar]
- Prullage JB, Cawthorne WG, Le Hir de Fallois LP, Timmons PR. 2011. Synergy between fipronil and amitraz in a Rhipicephalus sanguineus tick residual contact test. Experimental and Applied Acarology, 54, 173–176. [CrossRef] [PubMed] [Google Scholar]
- Ravera I, Altet L, Francino O, Sanchez A, Roldan W, Villanueva S, Ferrer L. 2013. Small Demodex populations colonize most parts of the skin of healthy dogs. Veterinary Dermatology, 24, 168–170. [CrossRef] [PubMed] [Google Scholar]
- Soulsby E. 1982. Helminths, Arthropods, and Protozoa of Domesticated Animals, 7th edn. Baillière Tindall: London. 809 pp. [Google Scholar]
Cite this article as: Fourie J, Dumont P, Halos L, Beugnet F & Pollmeier M: Efficacy of a topical application of Certifect® (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v) for the treatment of canine generalized demodicosis. Parasite, 2013, 20, 46.
All Tables
Individual Demodex counts for each dog at each evaluation and mean percentage of mite count reduction for each group.
Evaluation of the clinical signs associated with demodicosis and hair regrowth during the 3-month study period.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


