| Issue |
Parasite
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 47 | |
| Number of page(s) | 14 | |
| DOI | https://doi.org/10.1051/parasite/2025046 | |
| Published online | 29 July 2025 | |
Research Article
Monogeneans on exotic Indian freshwater fish. 8. Co-translocation of Cichlidogyrus tilapiae (Monogenea, Dactylogyridae) with pindani Chindongo socolofi (Cichliformes, Cichlidae): first report of this parasite genus in India within aquarium trade facilities
Monogènes des poissons d’eau douce exotiques en Inde. 8. Cotranslocation de Cichlidogyrus tilapiae (Monogenea, Dactylogyridae) avec le pindani Chindongo socolofi (Cichliformes, Cichlidae), première observation de ce genre de parasite en Inde dans des commerces d’aquariophilie
1
Department of Zoology, University of Lucknow, Uttar Pradesh 226 007, India
2
Institute of Evolutionary Science of Montpellier (ISEM), Centre National de la Recherche Scientifique (CNRS), Université de Montpellier, Institut de Recherche pour le Développement (IRD), 34095 Montpellier, France
3
Laboratory Biodiversity, Ecology and Genome, Faculty of Sciences, Mohammed V University in Rabat, 10000 Rabat, Morocco
4
Research Group Zoology: Biodiversity & Toxicology, Centre for Environmental Sciences, Hasselt University, 3590 Diepenbeek, Belgium
* Corresponding authors: antoine.pariselle@ird.fr (Antoine Pariselle); tripathi_amit@lkouniv.ac.in (Amit Tripathi)
Received:
7
May
2025
Accepted:
11
July
2025
The pindani, Chindongo socolofi (Cichliformes, Cichlidae) is a popular freshwater ornamental fish from Lake Malawi in Africa. Although identifying parasites associated with the global ornamental fish trade is critical for developing biosecurity practices, little is known about the parasite fauna of C. socolofi. Therefore, this study sought to determine what monogenean parasites C. socolofi harbours in India. Adult specimens of this host species were collected from various aquarium shops across the country between 2020 and 2022, and their gills were subjected to parasitological examination. Monogeneans were detected in five host specimens (22.7%) with low mean intensities (6.2 ± 3.8). They were identified as Cichlidogyrus tilapiae (Monogenea: Dactylogyridae) based on the presence of the following morphometric characteristics: two pairs of anchors, two auricles on the dorsal bar, a V-shaped ventral bar, and an accessory piece with a folded rim and a bent bifurcated tip. The morphological identification was confirmed by the sequence analysis of the specimen’s 18S-ITS1 gene regions and 28S rRNA genes to C. tilapiae from Paratilapia polleni (Cichliformes, Cichlidae) in Madagascar. This article is the first report on a species of Cichlidogyrus in India, found in aquarium shops, contributing to the growing list of known freshwater monogeneans that are being distributed globally via the ornamental fish trade. Additionally, it adds a new host species (C. socolofi) and geographic location (India, within aquarium trade) to the existing knowledge of C. tilapiae, a widespread and often co-introduced tropical fish parasite.
Résumé
Le pindani, Chindongo socolofi (Cichliformes, Cichlidae), est un poisson d’ornement d’eau douce populaire originaire du lac Malawi, en Afrique. Bien que l’identification des parasites associés au commerce mondial des poissons d’ornement soit essentielle au développement de pratiques de biosécurité, on sait peu de choses sur la faune parasitaire de C. socolofi. Par conséquent, cette étude visait à déterminer quels monogènes parasites C. socolofi héberge en Inde. Des spécimens adultes de cette espèce hôte ont été collectés dans divers magasins d’aquariophilie du pays entre 2020 et 2022, et leurs branchies ont été soumises à un examen parasitologique. Des monogènes ont été détectés chez cinq spécimens hôtes (22,7 %) avec de faibles intensités moyennes (6,2 ± 3,8). Ils ont été identifiés comme Cichlidogyrus tilapiae (Monogenea : Dactylogyridae) grâce à la présence des caractéristiques morphométriques suivantes : deux paires d’ancres, deux auricules sur la barre dorsale, une barre ventrale en V et une pièce accessoire avec un bord recourbé et une extrémité bifurquée et courbée. L’identification morphologique a été confirmée par l’analyse de la séquence des régions du gène 18S-ITS1 et des gènes de l’ARNr 28S du spécimen de C. tilapiae de Paratilapia polleni (Cichliformes, Cichlidae) à Madagascar. Cet article est le premier rapport sur la présence d’une espèce de Cichlidogyrus en Inde, dans des aquariums, et contribue à compléter la liste croissante des monogènes d’eau douce connus et introduits dans le monde entier via le commerce des poissons d’ornement. De plus, il ajoute une nouvelle espèce hôte (C. socolofi) et une nouvelle localisation géographique (l’Inde, dans des magasins d’aquariophilie) aux connaissances existantes sur C. tilapiae, un parasite répandu et souvent co-introduit chez les poissons tropicaux.
Key words: Parasites / Ornamental fish trade / 18S-ITS1 and 28S rRNA genes / Haplotype
Edited by: Jean-Lou Justine
© A. Tripathi et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cichlidogyrus Paperna, 1960 (Monogenea: Dactylogyridae) is the most species-rich African freshwater monogenean genus [37, 64]. It has 141 valid species [93] naturally parasitising primarily African cichlids (Cichlidae) and a few representatives of Cyprinodontidae Wagner, 1828 (Cyprinodontiformes) and Nandidae Bleeker, 1852 (Anabantiformes) [12, 20]. Some of these species have been identified as potentially pathogenic to fish, especially in aquaculture stocks [31, 56, 69]. Cichlidogyrus species, with few exceptions, are quite host-specific ([64], but also see [40]). An exception is Cichlidogyrus tilapiae Paperna, 1960. Since its first description from the Nile Tilapia, Oreochromis niloticus (Linnaeus 1758) (Cichliformes: Cichlidae) in Israel, C. tilapiae has been recorded in 31 different fish species in 27 countries across five continents, including Asia, Africa, North America, South America, and Australia (Table 1).
Global distribution of Cichlidogyrus tilapiae Paperna, 1960 for 27 countries and 31 host fishes.
Pindani, Chindongo (Pseudotropheus) socolofi (Johnson, 1974) (Cichliformes, Cichlidae) is native to Lake Malawi in Africa [18, 46], and is available in two colour variants – normal (Blue pindani) and albino (White pindani) [33]. Despite the importance of C. socolofi in the ornamental fish market [74], little is known about its parasite fauna. To our knowledge, only one study on parasitic infections of C. socolofi exists [11]. These researchers studied the parasites of cichlids imported via the aquarium trade in Türkiye and recorded the protozoan parasite Trichodina pediculus Ehrenberg, 1831.
This study aimed to establish whether C. socolofi is infected by monogenean parasites and, if so, whether they were co-translocated into India via the ornamental fish trade. We demonstrate the presence of C. tilapiae in post-quarantine populations of C. socolofi collected from Indian aquarium markets. This was accomplished first by morphological characterisation (structure and measurements of the sclerotised parts of the haptor and reproductive organs) and subsequently by molecular characterisation (Sanger sequencing of 18S rRNA gene-ITS1 region and 28S rRNA genes). This paper is part of a series on exotic and/or invasive monogenean parasites imported into India via the ornamental trade [81–87].
Materials and methods
Ethics
This study was approved by the institutional ethics committee of the University of Lucknow under the protocol numbers LU/AEC/ZOO/2019 and 19/I/2024/IAEC/LU.
Sample collection and examination
Between January 2020 and December 2022, 22 specimens of C. socolofi (total weight: 3.12–6.50 g; and total length: 4.5–8.0 cm) (Fig. 1) were collected from aquarium shops in Lucknow, New Delhi, and Kolkata, India. Fish were shipped to the laboratory the same day after they were packaged in polybags containing water and pure oxygen. Individual fish were euthanised with an overdose of tricaine methanesulfonate (MS-222 @ 150 mg/L; Sigma Aldrich Co., St. Louis, MO, USA), followed by exsanguination by the removal of gill arches. Half of the gill arches were initially fixed in hot (60 °C) distilled water to relax and heat-kill the specimens before they were transferred to 4% formalin for microscopy following Kritsky [36]. The other half was preserved in 95% ethanol for genetic analysis. Some of the gill arches were examined fresh with live worms. Monogeneans were later isolated from these gills using fine dissecting needles under a stereomicroscope (Leica Microsystems, Wetzlar, Germany). Fish specimens were identified morphologically with the help of the ICAR-National Bureau of Fish Genetic Resources (ICAR-NBFGR), a premier Indian institute on fish taxonomy, biology, and genomics.
 |
Figure 1 Freshly dead specimens of Chindongo socolofi (Johnson, 1974) examined for the present study. A. Blue pindani, B. White pindani. Photograph by Chawan Matey. |
Morphological analysis
Formalin-fixed worms were stained with either Gomori’s trichrome or Borax carmine and mounted in DPX (dibutylphthalate polystyrene xylene) for observing internal anatomy (permanent mounts); others were mounted in glycerine jelly or Hoyer’s medium for the study of sclerotised parts of the haptor and reproductive organs (temporary mounts). Additionally, some ethanol-preserved worms were treated for 20–30 min at 55 °C with 1.0 μL of digestion buffer (0.1 μl of solid tissue buffer and 0.9 μL proteinase K) (Quick DNATM Miniprep Plus Kit, ZYMO Research, Irvine, CA, USA) to digest the tissues surrounding their sclerotised parts.
The morphology of the sclerotised parts was examined under a light microscope (Leica DM4B) at a magnification of 100×, using an oil immersion lens with phase-contrast (PHA-CO) and differential interference contrast illumination. Photographs and measurements (in micrometres) were obtained using a digital camera (Leica DFC7000 T) and imaging analysis software (LAS X; Leica Microsystems Ltd.) attached to the light microscope. A composite line drawing plate was made from multiple parasite specimens using an Olympus BX-51 microscope drawing tube. Species were identified based on the morphological characters described in previous studies [14, 15, 35, 44, 57, 64]. The terminology and measurement of these characters followed Rahmouni et al. [70]. The prevalence and mean intensity of infection were calculated according to Bush et al. [8].
DNA extraction and amplification
Representative samples of ethanol-preserved specimens were morphologically identified as conspecific to the temporary and permanent mounts before being pooled in two groups (n = 3) by two collection sites (Lucknow and New Delhi) for gDNA isolation using a DNA extraction kit (Extracta DNA Prep for PCR-Tissue, Quantabio, Beverly, MA, USA), according to the manufacturer’s instructions. Partial fragments of 18S ribosomal RNA genes (18S) and internal transcribed spacer 1 (ITS1) clusters were amplified with the primers s1 [78] and ir8 [75]. Meanwhile, those of 28S ribosomal RNA genes were amplified with the primers c1 and d2 [23].
Polymerase chain reactions were performed in an automated thermal cycler (Himedia Laboratories, Thane, MH, India) with reaction mixtures (final volume 20 μL) containing 4 μL of distilled water, 10 μL of 2× PCR TaqMixture (Himedia Laboratories), 1 μL of 10 pmol/microliter of each primer, and 4 μL of DNA template. The amplification profile for the 18S rRNA gene-ITS1 region was as follows: initial denaturation at 95 °C for 3 min, then 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. The amplification profile for the 28S ribosomal RNA gene followed Šimková et al. [76]. The size of the PCR products (2 μL) was analysed by electrophoresis in 1.2% agarose gel prepared in 1× TAE buffer, prestained with 0.1 μL/mL 10,000× Sybr Safe in dimethyl sulfoxide (Invitrogen, Waltham, MA, USA), at 90 V for 30 min, and visualised and documented on a Bio-Print gel documentation imaging system (Vilber Lourmat, Collégien, France).
Sequence analysis
The PCR products were purified (on 1.5% agarose using a QIAquick PCR Purification Kit; QIAGEN, Germantown, MA, USA) and Sanger sequenced (on an ABI 3730xL automated sequencer; Applied Biosystems, Foster City, CA, USA) with PCR primers by Eurofins Genomics (Bengaluru, KA, India). SnapGene version 5.3 (https://www.snapgene.com) was used to manually quality-trim the successfully sequenced amplicons. Consensus sequences (18S-ITS1, 942 bp; 28S, 660 bp and 848 bp) were generated using the BioEdit Program [22].
Sequences, together with all sequences from the same markers and species retrieved from NCBI GenBank (Tables 2 and 3) were aligned using ClustalW [24] implemented in MEGA v.7 [38]. To obtain equal lengths for sequence analysis, they were trimmed to 687 bp (18S-ITS1) and 631 bp (28S). A median-joining network [3] was inferred for each marker using PopART [39].
Information on Cichlidogyrus tilapiae Paperna, 1960, including hosts, localities, and GenBank accession numbers of their 18S+ITS1 rRNA gene sequences (as retrieved from the NCBI database on December 04, 2024).
Information on Cichlidogyrus tilapiae Paperna, 1960, including hosts, localities, and GenBank accession numbers of their 28S rRNA gene sequences (as retrieved from the NCBI database on December 04, 2024).
As additional assessment for species-level identification, intraspecific genetic differences (also known as genetic distances) among different geographical isolates of C. tilapiae were computed from the same dataset. This was done using the Kimura two-parameter (K2P) model [34] of nucleotide substitution in MEGA 11 [79], with gaps treated as complete deletions.
Results
Thirty individuals of a single monogenean species – namely Cichlidogyrus tilapiae Paperna, 1960 – were collected from the gills of five specimens of C. socolofi, with a low prevalence (22.72%) and low infection intensities (7–13 worms/fish). Brief morphological and molecular data of this species are presented below.
Taxonomic summary
Phylum Platyhelminthes Minot, 1876
Superclass Neodermata Ehlers, 1985
Class Monogenea van Beneden, 1858
Family Dactylogyridae Yamaguti, 1963
Genus Cichlidogyrus Paperna, 1960
Cichlidogyrus tilapiae Paperna, 1960 (Figs. 2 and 3)
Type host and locality: Nile tilapia Oreochromis niloticus (Linnaeus, 1758) (Cichliformes: Cichlidae); Israel (Paperna 1960).
 |
Figure 2 Photomontage of light microscopy and phase contrast (PHACO) images of Cichlidogyrus tilapiae Paperna, 1960 from Chindongo socolofi (Johnson, 1974). A. anchor-bar complex and hooks, B and C. different configurations of male copulatory organ, D. vagina. Photograph by Amit Tripathi. |
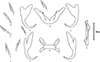 |
Figure 3 Line drawings of sclerotised structures of Cichlidogyrus tilapiae Paperna, 1960 from Chindongo socolofi (Johnson, 1974). A. ventral anchor, B. ventral bar, C. dorsal anchor, D. dorsal bar, E. male copulatory organ, F. hook (pairs i–vii). Scale bar = 20 μm. Figure by Amit Tripathi. |
Present host: Chindongo socolofi (Cichliformes, Cichlidae).
Present material and collection date: Aquarium shops in Lucknow (26.8467° N, 80.9462° E), New Delhi (28.6139° N, 77.2090° E), and Kolkata (22.5726° N, 88.3639° E), India; January 2020–December 2022.
Site of infection: Gills.
Infection parameters: Prevalence: 22.72% (5 out of 22 C. socolofi examined); Mean infection intensity: 6.2 ± 3.86 (7–13; n = 5).
Museum material: Five voucher specimens stained with Gomori’s trichrome or Borax carmine and mounted on glass slides in DPX (Smithsonian Institution, USA; USNM 1757684-1757688).
GenBank deposition: 18S-ITS1: 942 bp (MZ266637); 28S: 660 bp (MZ265190), 848 bp (PQ675652).
Morphological data
Cichlidogyrus is distinguished by a vas deferens that does not encircle the intestinal caecum, two pairs of anchors (one dorsal and one ventral), two transversal bars (a dorsal bar with two typical auricles and a V-shaped ventral bar), seven pairs of hooks, a sclerotised or non-sclerotised vagina and a sclerotised male copulatory organ comprising a male copulatory tube and (often but not always) an accessory piece [57, 88].
Our specimens presented nearly identical morphological features of sclerotised parts (both haptoral and reproductive ones) indicated in the original description [55] and subsequent redescriptions or illustrated records [14, 15, 35, 44] of C. tilapiae (Figs. 2 and 3) (Table 4). Only two minor discrepancies were observed in the morphometry of the haptoral armaments. First, our specimens had a slightly longer and deeper outer root of the dorsal anchor (4–6 μm), compared to their conspecifics. Second, variations were observed in the ranges of measurements of the ventral bar. Paperna [57], for example, measured the length of the ventral bar to be 34–98 μm, whereas Douëllou [14] measured it to be 26–33 μm (as we did), and Kritsky and Thatcher [35], Ergens [15], and Maneepitaksanti and Nagasawa [44] measured it to be 50–65 μm.
Comparative measurements (in μm) of reproductive organs and haptoral armaments of Cichlidogyrus tilapiae Paperna, 1960 from India (present study) and other geographical locations.
We were also able to locate the vagina in a single live specimen, which had gone unnoticed in previous studies on C. tilapiae. It resembled a short unsclerotised (muscular?) tube with a funnel-like opening at one end (Fig. 3D). We lost it quickly however, when the vitellaria burst out of the parasite body, killing it. Therefore, our identification of the vagina may not be conclusive and should be reconfirmed. Cichlidogyrus tilapiae has previously been adequately described/redescribed and, thus, does not need to be formally redescribed here.
Molecular data
The partial 18S rRNA gene-ITS1 region (942 bp) and 28S rRNA genes (660 bp and 848 bp) were sequenced from two pools of C. tilapiae specimens collected from C. socolofi in aquarium shops in India. Comparative analysis of these sequences against the NCBI GenBank non-redundant database using the “megablast” algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed “near perfect” matches for 28S rRNA (659/660 bp; 99.85% similar identity with a query coverage of 100%) and 18S rRNA-ITS1 (935/938 bp; 99.68% similar identity with a query coverage of 99%) to C. tilapiae from Paratilapia polleni Bleeker, 1868 (Cichliformes, Cichlidae) in Madagascar deposited in GenBank under the accession numbers MH767412 (28S) and MH767400 (18S-ITS1), respectively [77]. These findings suggest their conspecificity (Tables 5 and 6).
Intraspecific genetic distances (Kimura 2-parameter model with partial deletion option) and variations between our samples and conspecific references (most similar BLAST hits) of Cichlidogyrus tilapiae Paperna, 1960 based on 18S rRNA gene-ITS1 region.
Intraspecific genetic distances (Kimura 2-parameter model with partial deletion option) and variations between our samples and conspecific references (most similar BLAST hits) of Cichlidogyrus tilapiae Paperna, 1960 based on 28S rRNA gene.
The intraspecific genetic distances for 28S rRNA and 18S rRNA genes between four geographic isolates of C. tilapiae from different hosts and geographical locations were determined at 0%, indicating their conspecificity (Tables 5 and 6). The genetic distance for the ITS1 sequence, another marker with higher variability, was also determined between 0 and 0.002% (Table 5).
The haplotype network indicated that Indian haplotypes, for both markers, were widespread, and shared with conspecifics from both native and introduced populations (Fig. 4).
 |
Figure 4 Median-joining haplotype networks based on a 687 bp fragment of small subunit rDNA and the first Internal Transcribed Spacer (left) and 631 bp of large subunit rDNA (right) from the newly sequenced individuals of Cichlidogyrus tilapiae from India, aligned with all previously published sequences from this species. Genotypes are represented by circles, with the size of the circle correlating with the number of isolates displaying the respective genotype. Colours denote the countries of sampling localities. Genotypes are connected by lines indicating the number of mutations between them. |
Discussion
This paper is the first to document the presence of a member of Cichlidogyrus in India. In addition, C. socolofi has been identified as a new host species for C. tilapiae. This is also the first time a species of Cichlidogyrus is formally reported from a Malawi cichlid; members of the genus are known to occur on the lake’s cichlids but were mentioned without species-level identification [6]. Although there are a few variations in morphometrical data between different geographic isolates of C. tilapiae, we do not consider these differences to merit species-level separation. These variations may be attributable to differences in the host species [55], environmental factors [7], developmental stages [85, 90], individual variations within the species, or even the different fixation [16] or measuring methods employed thus far. For instance, just as we did, Douëllou [14] measured only one branch of the ventral bar following established norms of measurements for Cichlidogyrus [70]. Meanwhile, Kritsky and Thatcher [35] and Ergens [15] measured the total length in a “straight line extending between the two most distant parts”. Unfortunately, Paperna [57] and Maneepitaksanti and Nagasawa [44] did not specify their measurement methods.
While many publications have recorded only the occurrence of C. tilapiae without providing any morphometric data, those that have provided such data have shown a few variations in the sclerotised parts. For example, Paperna [57] found that both pairs of anchors were “about the same length”. However, all subsequent investigations have clearly shown that dorsal anchors are slightly larger than the ventral anchors. Paperna [57] also described and illustrated an accessory piece that terminated in a “bent bifurcated tip”, but this bifurcation has not been observed in any other studies. Furthermore, Ergens [15] and Douëllou [14] noted a small “groove on the base of dorsal anchor”, that no other researchers have reported. Ergens [15] also illustrated a small sliver-like structure on the outer roots of the ventral anchor, which has not been described or illustrated by other researchers.
Nonetheless, the distinctive morphology of the male copulatory organ, which lacks a heel and has a hook-shaped terminal end of the accessory piece, is consistent enough in all illustrations of C. tilapiae to be considered the most reliable diagnostic trait for identifying this species. This aligns with the notion that identification of Cichlidogyrus species is primarily based on the morphology of the reproductive hard parts [89].
Curiously, the 18S rRNA gene-ITS1 region and 28S rRNA gene sequences of C. tilapiae found in India differed from their conspecific references by only 3 bp and 1 bp, respectively (see above). Different phenotypes of C. tilapiae did not cluster monophyletically in the recent morphology-based phylogeny [51]. Therefore, we speculate that either C. tilapiae comprises a species complex of morphologically variable but closely related lineages [66] or that there are geographical variants of a single species.
The haplotype networks (Fig. 4) indicate that the haplotype of C. tilapiae found in India occurs widely throughout native and introduced host and parasite populations. Other markers than the ones used here, for example a sequence fragment of the cytochrome c oxidase subunit 1 gene, allow higher resolution distinction between populations of C. tilapiae [30] and may allow the identification of native and (co-)introduced strains of cichlid parasites [21].
Indian scenario
Since nothing is known about the monogenean fauna of C. socolofi in the wild, we cannot ascertain whether it is a natural host of C. tilapiae or whether it acquired it from other cichlids cohabiting in aquarium conditions. However, it is highly likely that C. socolofi is a regular host for C. tilapiae because the latter was consistently recovered over space (Lucknow, New Delhi, and Kolkata) and time (January 2020–December 2022). Chindongo socolofi is currently maintaining its self-sustaining populations in the country’s aquacultural ponds and has yet to be recorded in the wild. The potential negative impact of C. socolofi on India’s environment and/or economy will therefore depend on its ability to successfully establish, dominate, and expand in Indian waters. It has previously been hypothesised that the invasion success of a fish is linked to, amongst other things, favourable environmental conditions in the new habitat that are comparable to those in its native ranges (climate match theory; [1, 25]), and to the enemy release hypothesis [80]. Chindongo socolofi may be considered a potentially invasive fish species in this context because the climatic conditions in India, particularly in South India, are similar to those found in the native range of C. socolofi (southeastern Africa), including a tropical climate and a temperature range of 24–26 °C [18].
Should C. socolofi become invasive in Indian waters, the low host specificity of C. tilapiae (see above), combined with the native fish species’ lack of protective immunity against exotic parasites [72], could pose a serious biological invasion challenge. It is worth noting that C. tilapiae has already demonstrated its ability to switch from introduced cichlids to native hosts in destination environments, such as Vieja fenestrata (Günther, 1860) (Cichliformes, Cichlidae) (syn. = Paraneetroplus fenestratus) in Mexico [19], and Coptodon tholloni (Sauvage 1884) (Cichliformes, Cichlidae) in the Lower Congo Basin [29], and even non-cichlid hosts: Pachypanchax omalonotus (Duméril, 1861) (Cyprinodontiformes: Aplocheilidae) in Madagascar [77]. In fact, tilapia-infecting monogeneans have been proposed as the most ubiquitous tropical freshwater fish parasites globally, with C. tilapiae being one of the species most frequently reported as co-introduced with translocated tilapias [73]. However, to the best of our knowledge, this is the first report of this parasite from the ornamental fish trade.
The presence of C. tilapiae on C. socolofi highlights an additional challenge in India namely, illegal ornamental fish trafficking. The “Guidelines for import of ornamental fishes into India” [53] includes an “indicative list” of 92 exotic ornamental fish species that the Government of India has approved for import. Although C. socolofi is not on this list, it is widely available in Indian domestic trade ([67], this report).
Clearly, the fish were acquired illegally via international smuggling. Unfortunately, the “Guidelines” makes no clear or implicit declaration prohibiting the import of ornamental fish that are not on the “indicative list”, nor does it suggest that violators will face prosecution or even a fine. It simply states that “the import permit shall be cancelled forthwith and all the specimens imported destroyed without any notice to or permission of the importer”.
Given that the issue at hand involves not only fish trafficking but also the trafficking of accompanying (unidentified and often overlooked) parasites, merely cancelling import permits is a minor step toward protecting biodiversity and deterring traffickers. In reality, illegally imported exotic species are more likely to introduce parasites and diseases into the country because they bypass the import risk analysis and quarantine procedures of the importing country. Therefore, we recommend that the sale of a non-permitted ornamental fish species be treated as a criminal offence comparable to wildlife smuggling and implementing heavy penalties for this crime.
Acknowledgments
AT thanks Christoph Hahn (University of Graz, Austria) for a useful discussion on a previous version of the manuscript.
Funding
This work was fully supported by a project grant from the Science and Engineering Research Board (SERB), Government of India to AT (SERB–EMR/2017/003232). CM acknowledges the Ministry of Tribal Affairs, Government of India for providing her a PhD fellowship (202223-NFST-ARU-00531).
Conflicts of interest
The authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Agostinho AA, Suzuki HI, Fugi R, Alves DC, Tonella LH, Espindola LA. 2015. Ecological and life history traits of Hemiodus orthonops in the invasion process: looking for clues at home. Hydrobiologia, 746, 415–430. [Google Scholar]
- Arthur JR, Lumanlan-Mayo S. 1997. Checklist of the parasites of fishes of the Philippines. Rome: FAO. [Google Scholar]
- Bandelt H, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16, 37–48. [CrossRef] [PubMed] [Google Scholar]
- Bayoumy EM, El-Monem SA. 2012. Functional adaptation of branchial and stomach dactylogyrid monogenean: Cichlidogyrus and Enterogyrus isolated from Oreochromis niloticus, Proceedings of the 5th Global Fisheries & Aquaculture Research Conference, Egypt, 1 – 3 October, p. 353–360. [Google Scholar]
- Blahoua GK, Yao SS, Etile RND, N’Douba V. 2016. Distribution of gill Monogenean parasites from Oreochromis niloticus (Linnaeus, 1758) in man-made Lake Ayame I, Côte d’Ivoire. African Journal of Agricultural Research, 11, 117–129. [Google Scholar]
- Blais J, Rico C, van Oosterhout C, Cable J, Turner GF, Bernatchez L. 2007. MHC adaptive divergence between closely related and sympatric African cichlids. PLoS One, 2, e734. [Google Scholar]
- Brazenor AK, Saunders RJ, Miller TL, Hutson KS. 2018. Morphological variation in the cosmopolitan fish parasite Neobenedenia girellae (Capsalidae: Monogenea). International Journal of Parasitology, 48, 125–134. [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83, 575–583. [Google Scholar]
- Boungou M, Kabre GB, Marques A, Sawadogo L. 2008. Dynamics of population of five parasitic monogeneans of Oreochromis niloticus Linnaeus, 1757 in the dam of Loumbila and possible interest in intensive pisciculture. Pakistan Journal of Biological Sciences, 11, 1317–1323. [Google Scholar]
- Cavalcanti LD, Gouveia EJ, Leal FC, Figueiro CSM, Rojas SS, Russo MR. 2020. Responses of monogenean species to variations in abiotic parameters in tilapiculture. Journal of Helminthology, 94, e186. [Google Scholar]
- Celik SY, Korun J. 2018. Türkiye’ den Trichodinid Protozooan Trichodina heterodentata ve T. pediculus (Ciliophora: Trichodinidae) İçin Yeni Konak Kaydı. Kocatepe Veterinary Journal, 11, 245–254. [Google Scholar]
- Cruz-Laufer AJ, Artois T, Smeets K, Pariselle A, Vanhove MPM. 2021. The cichlid – Cichlidogyrus network: a blueprint for a model system of parasite evolution. Hydrobiologia, 848, 3847–3963. [CrossRef] [Google Scholar]
- da Graça RJ, Machado MH. 2007. Ocorrência e aspectos ecológicos de metazoários parasitos de peixes do Lago do Parque do Ingá, Maringá, Estado do Paraná. Acta Scientiarum: Biological Sciences, 29, 321–326. [Google Scholar]
- Douëllou L. 1993. Monogeneans of the genus Cichlidogyrus Paperna, 1960 (Dactylogyridae: Ancyrocephalinae) from cichlid fishes of Lake Kariba (Zimbabwe) with descriptions of five new species. Systematic Parasitology, 25, 159–186. [Google Scholar]
- Ergens R. 1981. Nine species of the genus Cichlidogyrus Paperna, 1960 (Monogenea: Ancyrocephalinae) from Egyptian fishes. Folia Parasitologica, 28, 205–214. [Google Scholar]
- Fankoua SO, Bitja Nyom AR, Bahanak DND, Bilong Bilong CF, Pariselle A. 2017. Influence of preservative and mounting media on the size and shape of monogenean sclerites. Parasitology Reseasrch, 116, 2277–2281. [Google Scholar]
- Ferdousi UK, Chandra KJ. 2002. New monogenean gill parasites of Oreochromis niloticus (Linnaeus, 1758) and Oreochromis mossambicus (Peters) (Osteichthyes, Cichlidae) from Mymensingh, Bangladesh, Rivista di Parassitolgia, 63, 49–60. [Google Scholar]
- Froese R, Pauly D (Eds.). 2024. FishBase. Available at www.fishbase.org (accessed 02 November 2024). [Google Scholar]
- García-Vásquez A, Razo-Mendivil U, Rubio-Godoy M. 2017. Triple trouble? Invasive poeciliid fishes carry the introduced tilapia pathogen Gyrodactylus cichlidarum in the Mexican highlands. Veterinary Parasitology, 235, 37–40. [Google Scholar]
- Geraerts M, Muterezi Bukinga F, Vanhove MPM, Pariselle A, Chocha Manda A, Verven E, Huyse T, Artois T. 2020. Six new species of Cichlidogyrus Paperna, 1960 (Platyhelminthes: Monogenea) from the gills of cichlids (Teleostei: Cichliformes) from the Lomami River Basin (DRC: Middle Congo). Parasites & Vectors, 13, 187. [CrossRef] [PubMed] [Google Scholar]
- Geraerts M, Huyse T, Barson M, Bassirou H, Bilong Bilong CF, Bitja Nyom AR, Cocha Manda A, Cruz-Laufer AJ, Kalombo Kabalika C, Kapepula Kasembele G, Muterezi Bukinga F, Njom S, Van Steenberge M, Artois T, Vanhove MPM. 2023. Sharing is caring? Barcoding suggests co-introduction of dactylogyrid monogeneans with Nile tilapia and transfer towards native tilapias in sub-Saharan Africa. International Journal for Parasitology, 53, 711–730. [CrossRef] [PubMed] [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Hassouna N, Michot B, Bachellerie JP. 1984. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Research, 12, 3563–3583. [CrossRef] [PubMed] [Google Scholar]
- Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680. [CrossRef] [PubMed] [Google Scholar]
- Howeth JG, Gantz CA, Angermeier PL, Frimpong EA, Hoff MH, Keller RP, Mandrak NE, Marchetti MP, Olden JD, Romagosa CM, Lodge DM. 2016. Predicting invasiveness of species in trade: climate match, trophic guild and fecundity influence establishment and impact of non-native freshwater fishes. Diversity and Distributions, 22, 148–160. [Google Scholar]
- Jimenez-Garcia MI, Vidal-Martinez VM, Lopez-Jimenez S. 2001. Monogeneans in introduced and native cichlids in México: Evidence for transfer. Journal of Parasitology, 87, 907–909. [Google Scholar]
- Jorissen MWP, Pariselle A, Huyse T, Vreven EJ, Snoeks J, Decru E, Kusters T, Wamuini Lunkayilakio S, Muterezi Bukinga F, Artois T, Vanhove MPM. 2018. Six new dactylogyrid species (Platyhelminthes, Monogenea) from the gills of cichlids (Teleostei, Cichliformes) from the Lower Congo Basin. Parasite, 25, 64. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Jorissen MWP, Pariselle A, Huyse T, Vreven EJ, Snoeks J, Volckaert FAM, Chocha Manda A, Kapepula Kasembele G, Artois T, Vanhove MPM. 2018. Diversity and host specificity of monogenean gill parasites (Platyhelminthes) of cichlid fishes in the Bangweulu-Mweru ecoregion. Journal of Helminthology, 92, 417–437. [CrossRef] [PubMed] [Google Scholar]
- Jorissen MWP, Huyse T, Pariselle A, Wamuini Lunkayilakio S, Muterezi Bukinga F, Chocha Manda A, Kapepula Kasembele G, Vreven EJ, Snoeks J, Decru E, Artois T, Vanhove MPM. 2020. Historical museum collections help detect parasite species jumps after tilapia introductions in the Congo Basin. Biological Invasions, 22, 2825–2844. [Google Scholar]
- Jorissen MWP, Vanhove MPM, Pariselle A, Snoeks J, Vreven EJ, Šimková A, Wamuini Lunkayilakio S, Chocha Manda A, Kapepula Kasembele G, Muterezi Bukinga F, Artois T, Huyse T. 2022. Molecular footprint of parasite co-introduction with Nile tilapia in the Congo Basin. Organisms Diversity & Evolution, 22, 1003–1019. [CrossRef] [Google Scholar]
- Kabata Z. 1985. Parasites and diseases of fish cultured in the tropics. London and Philadelphia: Taylor and Francis. [Google Scholar]
- Kapepula Kasembele G, Chocha Manda A, Abwe E, Pariselle A, Muterezi Bukinga F, Huyse T, Jorissen MWP, Vreven EJWMN, Luus-Powell WJ, Smit WJ, Sara JR. 2023. First record of monogenean fish parasites in the Upper Lufira River Basin (Democratic Republic of Congo): dactylogyrids and gyrodactylids infesting Oreochromis mweruensis, Coptodon rendalli and Serranochromis macrocephalus (Teleostei: Cichlidae). Parasites & Vectors, 16, 48. [CrossRef] [PubMed] [Google Scholar]
- Karadal O, Guroy D. 2015. Effect of albinism on reproductive performance on cichlid fish: Example of powder blue and snow white (Pseudotropheus socolofi) cichlids. Ege Journal of Fisheries and Aquatic Sciences, 3, 159–163. [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. [CrossRef] [PubMed] [Google Scholar]
- Kritsky DC, Thatcher VE. 1974. Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. Journal of Helminthology, 48, 59–66. [CrossRef] [PubMed] [Google Scholar]
- Kritsky DC. 2017. Dactylogyrids (Monogenoidea) infecting the gill lamellae of some beloniform fishes from Moreton Bay, Queensland, Australia, with a redescription of Hareocephalus thaisae Young, 1969 and descriptions of six new species of Hemirhamphiculus Bychowsky & Nagibina, 1969. Systematic Parasitology, 95, 33–54. [Google Scholar]
- Kuchta R, Basson L, Cook C, Fiala I, Řehulková E, Bartošová-Sojková P, Přikrylová I, Seifertová M, Kudlai O, Francová K, Scholz T, Smit N, Sures B, Kvach Y, Mašová S, Hadfield K. 2018. Part 4: a systematic survey of the parasites of freshwater fishes in Africa, in A guide to the parasites of African freshwater fishes, Scholz T, Vanhove MPM, Smit N, Jayasundera Z, Gelnar M, Editors, Royal Belgian Institute of Natural Sciences, Abc Taxa, p. 135–402. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. [CrossRef] [PubMed] [Google Scholar]
- Leigh JW, Bryant D. 2015. PopART: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6, 1110–1116. [CrossRef] [Google Scholar]
- Le Roux LE, Avenant-Oldewage A. 2010. Check-list of the genus Cichlidogyrus (Monogenea). African Journal of Aquatic Sciences, 35, 21–36. [Google Scholar]
- Lim SY, Ooi AL, Wong WL. 2016. Gill monogeneans of Nile tilapia (Oreochromis niloticus) and red hybrid tilapia (Oreochromis spp.) from the wild and fish farms in Perak, Malaysia: infection dynamics and spatial distribution. SpringerPlus, 5, 1609. [Google Scholar]
- Madanire-Moyo GN, Matla MM, Olivier PAS, Luus-Powell WJ. 2011. Population dynamics and spatial distribution of monogeneans on the gills of Oreochromis mossambicus (Peters, 1852) from two lakes of the Limpopo River system, South Africa. Journal of Helminthology, 85, 146–152. [Google Scholar]
- Madanire-Moyo GN, Luus-Powell WJ, Olivier PAS. 2012. Diversity of metazoan parasites of the Mozambique tilapia, Oreochromis mossambicus (Peters, 1852), as indicators of pollution in the Limpopo and Olifants River systems. Onderstepoort Journal of Veterinary Research, 79, 1–9. [Google Scholar]
- Maneepitaksanti W, Nagasawa K. 2012. Monogeneans of Cichlidogyrus Paperna, 1960 (Dactylogyridae), gill parasites of tilapias, from Okinawa Prefecture, Japan. Journal of Biogeography, 14, 111–119. [Google Scholar]
- Maneepitaksanti W, Worananthakij W, Sriwilai P, Laoprasert T. 2014. Identification and distribution of gill monogeneans from Nile tilapia and red tilapia in Thailand. Veterinary Integrative Sciences, 12, 57–68. [Google Scholar]
- Maréchal C. 1991. Pseudotropheus, in Check-list of the freshwater fishes of Africa (CLOFFA), vol. 4, Daget J, Gosse JP, Teugels GG, Thys van den Audenaerde DFE, Editors, ISNB, Brussels; MRAC, Tervuren and ORSTOM, Paris, pp. 401–415. [Google Scholar]
- Mendlová M, Pariselle A, Vyskočilová M, Šimková A. 2010. Molecular phylogeny of monogeneans parasitizing African freshwater Cichlidae inferred from LSU rDNA sequences. Parasitology Research, 107, 1405–1413. [CrossRef] [PubMed] [Google Scholar]
- Mendlová M, Desdevises Y, Civáňová K, Pariselle A, Šimková A. 2012. Monogeneans of West African cichlid fish: evolution and cophylogenetic interactions. PLoS One, 7, e37268. [CrossRef] [PubMed] [Google Scholar]
- Mendoza-Franco EF, Vidal-Martínez VM, Cruz-Quintana Y, Prats León FL. 2006. Monogeneans on native and introduced freshwater fishes from Cuba with the description of a new species of Salsuginus Beverley-Burton, 1984 from Limia vittata (Poeciliidae). Systematic Parasitology, 64, 181–190. [Google Scholar]
- Mendoza-Franco EF, Caspeta-Mandujano JM, Osorio MT. 2018. Ecto- and endo-parasitic monogeneans (Platyhelminthes) on cultured freshwater exotic fish species in the state of Morelos, South-Central Mexico. Zookeys, 776, 1–12. [Google Scholar]
- Moons T, Kmentová N, Pariselle A, Artois T, Bert W, Vanhove MPM, Cruz-Laufer AJ. 2023. All quiet on the western front? The evolutionary history of monogeneans (Dactylogyridae: Cichlidogyrus, Onchobdella) infecting a West and Central African tribe of cichlid fishes (Chromidotilapiini). Parasite, 30, 25. [Google Scholar]
- Natividad JM, Bondad-Reantaso MG, Arthur JR. 1986. Parasites of Nile Tilapia (Oreochromis niloticus) in the Philippines, in Proceedings of the first Asian Fisheries Forum, Maclean JL, Dizon LB, Hosillos LV, Editors, Asian Fisheries Society: Philippines. p. 255–259. [Google Scholar]
- NFDB. 2015–2020. Sanitary protocol for import of ornamental fishes into India. Available at https://nfdb.gov.in/welcome/guidelines (accessed 23 June 2024). [Google Scholar]
- Olivier PAS, Luus-Powell WJ, Saayman JE. 2009. Report on some monogenean and clinostomid infestations of freshwater fish and waterbird hosts in Middle Letaba Dam, Limpopo Province, South Africa. Onderstepoort Journal of Veterinary Research, 76, 187–199. [Google Scholar]
- Olstad K, Bachmann L, Bakke TA. 2009. Phenotypic plasticity of taxonomic and diagnostic structures in gyrodactylosis-causing flatworms (Monogenea, Platyhelminthes). Parasitology, 136, 1305–1315. [CrossRef] [PubMed] [Google Scholar]
- Paladini G, Longshaw M, Gustinelli A, Shinn AP. 2017. Parasitic diseases in aquaculture: their biology, diagnosis and control, in Diagnosis and control of diseases of fish and shellfish, Austin BA, Newaj-Fyzul A, Editors, John Wiley & Sons, p. 37–107. [Google Scholar]
- Paperna I. 1960. Studies on monogenetic trematodes in Israel. 2. Monogenetic trematodes of cichlids. Bamidgeh, 12, 20–33. [Google Scholar]
- Paperna I. 1965. Monogenetic Trematodes collected from fresh water fish in southern Ghana. Bamidgeh, 17, 107–115. [Google Scholar]
- Paperna I. 1968. Monogenetic trematodes collected from freshwater fish in Ghana. Second report. Bamidgeh, 20, 88–99. [Google Scholar]
- Paperna I. 1969. Monogenetic Trematodes of the fish of the Volta basin and South Ghana. Bulletin de l’Institut Français d’Afrique Noire, 31, 840–880. [Google Scholar]
- Paperna I, Thurston JP. 1969. Monogentic trematodes collected from cichlid fish in Uganda including the description of five new species of Cichlidogyrus. Revue de Zoologie et de Botanique Africaines, 79, 15–33. [Google Scholar]
- Paperna I. 1979. Monogenea of inland water fishes of Africa. Musée Royal de l’Afrique Centrale, Tervuren, Serie No. 8, 1–127. [Google Scholar]
- Paredes-Trujillo A, Velazquez-Abunader I, Torres-Irineo E, Romero D, Vidal-Martinez VM. 2016. Geographical distribution of protozoan and metazoan parasites of farmed Nile tilapia Oreochromis niloticus (L.) (Perciformes: Cichlidae) in Yucatan, Mexico. Parasites & Vectors, 9, 66. [Google Scholar]
- Pariselle A, Euzet L. 2009. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema, 31, 849–898. [Google Scholar]
- Pariselle A, Bitja Nyom AR, Bilong Bilong CF. 2013. Checklist of the ancyrocephalids (Monogenea) parasitizing Tilapia species in Cameroon, with the description of three new species. Zootaxa, 3599, 078–086. [Google Scholar]
- Pouyaud L, Desmarais E, Deveney M, Pariselle A. 2006. Phylogenetic relationships among monogenean gill parasites (Dactylogyridea, Ancyrocephalidae) infesting tilapiine hosts (Cichlidae): systematic and evolutionary implications. Molecular Phylogenetics and Evolution, 38, 241–249. [CrossRef] [PubMed] [Google Scholar]
- Premdass K, Lekeshmanaswamy M, Devi AK, Vasuki CA. 2016. Studies on the biodiversity of freshwater ornamental fishes, Tamilnadu, India. International Journal of Zoology and Applied Biosciences, 1, 15–26. [Google Scholar]
- Prieto TA, Vinjoy CM, Fajer AE. 1985. Cichlidogyrus sclerosus (Monogenea: Ancyrocepalinidae) en Tilapia hornorum × Tilapia mossambica (perca dorada) en cultivo intensivo. Revista de Salud Animal, 7, 291–295. [Google Scholar]
- Pugachev ON, Gerasev PI, Gussev AV, Ergens R, Khotenowsky I. 2009. Guide to Monogenoidea of freshwater fish of Palaearctic and Amur regions. Milan: Ledizione-Ledi Publishing. [Google Scholar]
- Rahmouni C, Vanhove MPM, Šimková A. 2018. Seven new species of Cichlidogyrus Paperna, 1960 (Monogenea: Dactylogyridae) parasitizing the gills of Congolese cichlids from northern Lake Tanganyika. PeerJ, 6, e5604. [CrossRef] [PubMed] [Google Scholar]
- Rindoria NM, Mungai LK, Yasindi AW, Otachi EO. 2016. Gill monogeneans of Oreochromis niloticus (Linnaeus, 1758) and Oreochromis leucostictus (Trewavas, 1933) in Lake Naivasha, Kenya. Parasitology Research, 115, 1501–1508. [CrossRef] [PubMed] [Google Scholar]
- Schmid-Hempel P. 2011. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford: Oxford University Press. [Google Scholar]
- Shinn AP, Avenant-Oldewage A, Bondad-Reantaso MG, Cruz-Laufer A, García Vásquez A, Hernández-Orts JS, Kuchta R, Longshaw M, Metselaar M, Pariselle A, Pérez-Ponce de León G, Pradhan PK, Rubio Godoy M, Sood N, Vanhove MPM, Deveney MR. 2023. A global review of problematic and pathogenic parasites of farmed tilapia. Reviews in Aquaculture, 15, 92–153. [CrossRef] [Google Scholar]
- Smith MP. 2000. Lake Malawi cichlids. New York: Barron’s Educational Series. [Google Scholar]
- Šimková A, Plaisance L, Matějusová I, Morand S, Verneau O. 2003. Phylogenetic relationships of the Dactylogyridae Bychowsky, 1933 (Monogenea: Dactylogyridea): the need for the systematic revision of the Ancyrocephalinae Bychowsky, 1937. Systematic Parasitology, 54, 1–11. [CrossRef] [PubMed] [Google Scholar]
- Šimková A, Matějusova I, Cunningham C. 2006. A molecular phylogeny of the Dactylogyridae sensu Kritsky & Boeger (1989) (Monogenea) based on the D1–D3 domains of large subunit Rdna. Parasitology, 133, 43–53. [CrossRef] [PubMed] [Google Scholar]
- Šimková A, Rehulkova E, Rasoloariniaina JR, Jorissen MWP, Scholz T, Faltynkova A, Masova S, Vanhove MPM. 2019. Transmission of parasites from introduced tilapias: a new threat to endemic Malagasy ichthyofauna. Biological Invasions, 21, 803–819. [CrossRef] [Google Scholar]
- Sinnappah ND, Lim LHS, Rohde K, Tinsley R, Combes C, Verneau O. 2001. A paedomorphic parasite associated with a neotenic amphibian host: phylogenetic evidence suggests a revised systematic position for Sphyranuridae within anuran and turtle polystomatoineans. Molecular Phylogenetics and Evolution, 18, 189–201. [CrossRef] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. 2021. MEGA 11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. [CrossRef] [PubMed] [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. 2003. Introduced species and their missing parasites. Nature, 421, 628–630. [CrossRef] [PubMed] [Google Scholar]
- Tripathi A, Agrawal N, Srivastava N. 2010. Monogenoidea on exotic Indian freshwater fishes. 1. A new geographical record of Sciadicleithrum iphthimum Kritsky, Thatcher, and Boeger, 1989 (Dactylogyridae) with the first description of its egg. Comparative Parasitology, 77, 83–86. [CrossRef] [Google Scholar]
- Tripathi A. 2014. The invasive potential of parasitic monogenoids (Platyhelminthes) via the aquarium fish trade: an appraisal with special reference to India. Reviews in Aquaculture, 5, 1–15. [Google Scholar]
- Tripathi A, Rajvanshi S, Agrawal N. 2014. Monogenoidea on exotic Indian freshwater fishes. 2. Range expansion of Thaparocleidus caecus and T. siamensis (Dactylogyridae) by introduction of striped catfish Pangasianodon hypophthalmus (Pangasiidae). Helminthologia, 51, 23–30. [CrossRef] [Google Scholar]
- Tripathi A. 2015. Monogenoidea on exotic Indian freshwater fish. 3. Are Indian guidelines for importation of exotic aquarium fish useful and can they be implemented; The case of Neotropical Gussevia spiralocirra Kohn and Paperna, 1964. Current Science, 108, 2101–2105. [Google Scholar]
- Tripathi A, Matey C, Agarwal N. 2022. Monogenoidea on exotic Indian freshwater fish. 4. Dactylogyrus minutus from Platinum Ogon, an ornamental variety of the common carp Cyprinus carpio (Cypriniformes, Cyprinidae). BioInvasions Record, 11, 510–523. [CrossRef] [Google Scholar]
- Tripathi A, Matey C. 2023. Monogenea on exotic Indian freshwater fish. 5. First report of pathogenic Gussevia asota (Platyhelminths) from Oscar Astronotus ocellatus (Agassiz 1831) (Perciformes: Cichlidae). Zootaxa, 5231, 052–064. [Google Scholar]
- Tripathi A, Matey C, Buchmann K, Hahn C. 2025. Monogeneans on exotic Indian freshwater fish. 7. Results of a national study on ornamental fishes from 2019–2022. Parasite, 32, 28. [Google Scholar]
- Vanhove MP, Volckaert FA, Pariselle A. 2011. Ancyrocephalidae (Monogenea) of Lake Tanganyika: I: Four new species of Cichlidogyrus from Ophthalmotilapia ventralis (Teleostei: Cichlidae), the first record of this parasite family in the basin. Zoologia, 28, 253–263. [Google Scholar]
- Vignon M, Pariselle A, Vanhove MPM. 2011. Modularity in attachment organs of African Cichlidogyrus (Platyhelminthes: Monogenea: Ancyrocephalidae) reflects phylogeny rather than host specificity or geographic distribution. Biological Journal of the Linnean Society, 102, 694–706. [CrossRef] [Google Scholar]
- Villar-Torres M, Montero FE, Merella P, Garippa G, Cherchi S, Raga JA, Repullés-Albelda A. 2022. From development to taxonomy: the case of Sciaenacotyle pancerii (Monogenea: Microcotylidae) in the Mediterranean meagre. Parasitology, 149, 1695–1701. [Google Scholar]
- Wanderson PMF, Ligia NR, Marcia DRD, Renata MGB, Montagner D, Tavares-Dias M. 2012. Protozoan and metazoan parasites of Nile tilapia Oreochromis niloticus cultured in Brazil. Revista MVZ Córdoba, 17, 2812–2819. [Google Scholar]
- Wilson JR, Saunders RJ, Hutson KS. 2019. Parasites of the invasive tilapia Oreochromis mossambicus: evidence for co-introduction. Aquatic Invasions, 14, 332–349. [CrossRef] [Google Scholar]
- WoRMS. 2025. Cichlidogyrus Paperna, 1960. Available at https://www.marinespecies.org/aphia.php?p=taxdetails&id=517932 (accessed 30 January 2025). [Google Scholar]
- Zhang S, Zhi T, Xu X, Zheng Y, Bilong-Bilong CF, Pariselle A, Yang T. 2019. Monogenean fauna of alien tilapias (Cichlidae) in South China. Parasite, 26, 4. [Google Scholar]
Cite this article as: Tripathi A, Matey C, Pariselle A & Vanhove MPM. 2025. Monogeneans on exotic Indian freshwater fish. 8. Co-translocation of Cichlidogyrus tilapiae (Monogenea, Dactylogyridae) with pindani Chindongo socolofi (Cichliformes, Cichlidae): first report of this parasite genus in India within aquarium trade facilities. Parasite 32, 47. https://doi.org/10.1051/parasite/2025046.
All Tables
Global distribution of Cichlidogyrus tilapiae Paperna, 1960 for 27 countries and 31 host fishes.
Information on Cichlidogyrus tilapiae Paperna, 1960, including hosts, localities, and GenBank accession numbers of their 18S+ITS1 rRNA gene sequences (as retrieved from the NCBI database on December 04, 2024).
Information on Cichlidogyrus tilapiae Paperna, 1960, including hosts, localities, and GenBank accession numbers of their 28S rRNA gene sequences (as retrieved from the NCBI database on December 04, 2024).
Comparative measurements (in μm) of reproductive organs and haptoral armaments of Cichlidogyrus tilapiae Paperna, 1960 from India (present study) and other geographical locations.
Intraspecific genetic distances (Kimura 2-parameter model with partial deletion option) and variations between our samples and conspecific references (most similar BLAST hits) of Cichlidogyrus tilapiae Paperna, 1960 based on 18S rRNA gene-ITS1 region.
Intraspecific genetic distances (Kimura 2-parameter model with partial deletion option) and variations between our samples and conspecific references (most similar BLAST hits) of Cichlidogyrus tilapiae Paperna, 1960 based on 28S rRNA gene.
All Figures
 |
Figure 1 Freshly dead specimens of Chindongo socolofi (Johnson, 1974) examined for the present study. A. Blue pindani, B. White pindani. Photograph by Chawan Matey. |
| In the text | |
 |
Figure 2 Photomontage of light microscopy and phase contrast (PHACO) images of Cichlidogyrus tilapiae Paperna, 1960 from Chindongo socolofi (Johnson, 1974). A. anchor-bar complex and hooks, B and C. different configurations of male copulatory organ, D. vagina. Photograph by Amit Tripathi. |
| In the text | |
 |
Figure 3 Line drawings of sclerotised structures of Cichlidogyrus tilapiae Paperna, 1960 from Chindongo socolofi (Johnson, 1974). A. ventral anchor, B. ventral bar, C. dorsal anchor, D. dorsal bar, E. male copulatory organ, F. hook (pairs i–vii). Scale bar = 20 μm. Figure by Amit Tripathi. |
| In the text | |
 |
Figure 4 Median-joining haplotype networks based on a 687 bp fragment of small subunit rDNA and the first Internal Transcribed Spacer (left) and 631 bp of large subunit rDNA (right) from the newly sequenced individuals of Cichlidogyrus tilapiae from India, aligned with all previously published sequences from this species. Genotypes are represented by circles, with the size of the circle correlating with the number of isolates displaying the respective genotype. Colours denote the countries of sampling localities. Genotypes are connected by lines indicating the number of mutations between them. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


