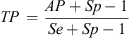| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 38 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/parasite/2024038 | |
| Published online | 09 July 2024 | |
Research Article
Prevalence and risk factors of Toxoplasma gondii infection in dairy cattle from the Western Region of Thailand
Prévalence et facteurs de risque de l’infection à Toxoplasma gondii chez les bovins laitiers de la région occidentale de la Thaïlande
1
Department of Large Animal and Wildlife Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen, Nakhon Pathom 73140, Thailand
2
Department of Pathology, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen, Nakhon Pathom 73140, Thailand
* Corresponding author: theera.r@ku.ac.th
Received:
2
February
2024
Accepted:
17
June
2024
In total, 901 dairy cow sera and data were collected from 51 farms in Nakhon Pathom, Ratchaburi and Kanchanaburi provinces (Western Region of Thailand). Serum samples were processed via the multispecies ELISA method to detect IgG antibodies against Toxoplasma gondii infection. The results demonstrated that the calculated true prevalence was 1.48% (95% CI, 0.64–2.75%) for the individual-level and 29.41% (95% CI, 18.71–43%) for the farm-level. The univariate risk factor analysis showed that the number of total owned cats, the presence of stray cats, and the frequency of cleaning per day were significant factors (p < 0.2). These three factors were subjected to logistic regression analysis, and the results revealed that the frequency of cleaning farms per day was a potential risk factor for T. gondii-seropositive farms (OR = 2.745, 95% CI, 1.15–8.69, p = 0.02). The frequency of cleaning might increase the T. gondii oocyst distribution within the barn area, thus increasing the possibility of infection. Our findings show that T. gondii continues to circulate in the dairy cow population in the western part of Thailand. The presence of cats on farms was not found to be associated with T. gondii infection, but the high frequency of cleaning the floor was, and contributed to the potential risk of infection.
Résumé
Au total, 901 sérums de vaches laitières et des données ont été collectés dans 51 fermes des provinces de Nakhon Pathom, Ratchaburi et Kanchanaburi (région occidentale de la Thaïlande). Les échantillons de sérum ont été traités via la méthode ELISA multi-espèces pour détecter les anticorps IgG contre l’infection à Toxoplasma gondii. Les résultats ont démontré que la prévalence réelle calculée était de 1,48 % (IC à 95 %, 0,64–2,75 %) au niveau individuel et de 29,41 % (IC à 95 %, 18,71–43 %) au niveau des exploitations. L’analyse factorielle a montré que le nombre total de chats possédés, la présence de chats errants et la fréquence quotidienne de nettoyage étaient des facteurs significatifs (p < 0,2). Ces trois facteurs ont été soumis à une analyse de régression logistique et les résultats ont révélé que la fréquence quotidienne de nettoyage des exploitations était un facteur de risque potentiel pour les exploitations séropositives à T. gondii (OR = 2,745, IC à 95 % = 1,15–8,69, p = 0,02). La fréquence du nettoyage pourrait favoriser la répartition des oocystes de T. gondii dans les étables, augmentant ainsi le risque d’infection. Nos résultats indiquent que T. gondii continue de circuler dans la population de vaches laitières de l’ouest de la Thaïlande. La présence de chats dans les fermes n’a pas été associée à l’infection à T. gondii, mais la fréquence élevée du nettoyage du sol l’était et contribuait au risque potentiel d’infection.
Key words: Dairy cattle / Risk factor / Serology / Toxoplasma gondii / Thailand
© N. Wannapong et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Toxoplasma gondii is an obligate intracellular protozoan that causes toxoplasmosis both in humans and mammals. The important clinical signs are abortion and neurological syndrome, which impact public health and the livestock industry. Hosts become infected by ingesting contaminated oocysts in food or water. Additionally, carnivorous hosts can be infected by consuming tissue cysts of the parasite in raw meat. The life cycle of T. gondii consists of both asexual and sexual cycles. The feline serves as the only definitive host, developing the sexual cycle by producing and shedding of un-sporulated oocysts into the environment via feces. Consequently, the sporulation of oocysts enables the protozoa to survive for long periods, even in extreme conditions due to the multilayered structure of the oocyst wall [23, 49]. The asexual cycle involves protozoan multiplication and differentiation between tachyzoites and bradyzoites. During the acute phase, tachyzoites invade enterocytes and subsequently enter the blood circulation. From there, the parasites disseminate throughout the body, infecting and multiplying within various host organs. The multiplication of parasites is decreased by the host immune response, which triggers tachyzoites to differentiate into bradyzoites and develop into tissue cysts or chronic stage. Conversely, when an infected host loses immune function, bradyzoites in tissue cysts can differentiate back to tachyzoites. This stage allows the parasite to disseminate throughout the body, causing re-emergence of the acute stage. Additionally, T. gondii infection can induce retinitis, myocarditis and placentitis, which can cause abortion in both humans and animals, including cattle. In pregnant hosts, fetuses may be infected through congenital transmission of tachyzoites [38].
The gold standard for T. gondii diagnosis is the dye test or Sabin–Feldman dye, which is an antibody detection method. However, it has disadvantages such as high cost and the use of live organisms, posing a human hazard. Other serological methods recommended for screening in survey studies include the indirect fluorescent antibody test (IFAT), modified agglutination test (MAT), indirect hemagglutination test (IHA), and latex agglutination test (LAT). Although these methods are convenient to perform, the results are interpreted visually, and individual variation may be a concern. One suitable technique for epidemiological studies is enzyme-linked immunosorbent assay (ELISA) due to its simplicity for mass screening surveys and accurate results read by a spectrophotometer. Viable parasites are detected by bioassay, with inoculation in a mouse or cat. Consequently, molecular techniques for genomic detection are necessary to confirm infection. These are time consuming processes requiring high biosecurity level in laboratories, making them unsuitable for survey studies [26, 43].
Dairy cattle are intermediate hosts of T. gondii. They become infected by ingesting contaminated oocysts in feed and water. The protozoa are then transferred to other hosts through the consumption of milk and undercooked meat from infected cattle [48]. Most studies have reported that cattle are resistant to T. gondii, and often infected pregnant cattle deliver normal calves and develop antibodies [9]. In addition, a study of pregnant cows (n = 4) showed that 50% of cows aborted after subcutaneous inoculation with tachyzoites [51]. In Thailand, the most recent survey was carried out 10 years ago and found a 9.42% seroprevalence in dairy cattle [20]. The western region is characterized by a high density of dairy cattle in Thailand, where the seroprevalence of T. gondii was 7% in 2008 [4]. Infection with T. gondii is not a specific clinical sign until cattle exhibit signs of fetal abortion and stillbirth. This is why it is difficult to diagnose toxoplasmosis in cattle. In addition, approximately one third of the global human population has been exposed to T. gondii [5, 38]. The foodborne nature of T. gondii via beef has not been confirmed, but viable protozoa have been detected from samples collected at slaughterhouses [32]. Although disease transmission from cattle to humans was not conclusively confirmed, T. gondii infection in cattle cannot be ruled out as a public health problem [10].
To prevent T. gondii infection in dairy cattle, several studies have investigated both risk and protective factors. Many findings suggest that prevention of contamination with oocysts is key. It is crucial to prohibit cats form accessing farm areas, including feed and water storage areas [19, 44]. Additionally, T. gondii infection is not a required test before animal transportation, and recently acquired animals should be screen tested and imported from reputable suppliers [3]. Differences in farm locations, management systems, and farmer activities are important considerations when implementing prevention strategies. This study aimed to determine the seroprevalence, spatial distribution, and risk factors for T. gondii infection in dairy cattle from the Western Region of Thailand. Understanding disease epidemiology is important for designing strategies for the control and prevention of infection.
Materials and methods
Ethics
This study was carried out with approval from the Committee for Animal Care and Use for Scientific Research, Kasetsart University, Thailand (ACKU64-VET-039). The farm owners gave permission for collection of samples and data.
Study area, sample collection, and processing
This study was carried out in three Western regions with the highest densities of dairy cows, namely Ratchaburi, Kanchanaburi and Nakhonpathom provinces (Fig. 1). The 51 farms were selected from members of the Kasetsart University Veterinary Teaching Hospital Kamphaeng Saen and Nong Pho, where mobile cinical services are provided in this area. The sample size of the seroprevalence study was calculated by the ProMESA program (version 2.3.0.2) via the estimated sample size–stratified random sample method. The calculation of the sample size required the value of the expected prevalence in each province, so we followed the 2010 study in Thailand; this value was 10% of the total T. gondii seroprevalence [20]. The population size in each province was calculated according to Department of Livestock data. In addition, the sample size (n) was calculated with an acceptable relative error of 0.2 and a 95% confidence interval following this formula.
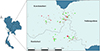 |
Figure 1 Study area in three provinces in the western part of Thailand. Geographical distribution of the dairy farms in this area, including T. gondii seropositive farms (red circles) and negative farms (green circles). |
The calculated sample sizes were 388, 272, and 194 blood samples collected from Ratchaburi, Kanchanaburi and NakhonPathom, respectively. Then, the number of samples from each farm was estimated by the method of Thrusfield [47] for the detection of disease in a group of animals. The requested sample sizes for each farm were calculated using the following formula [47]:
Approximately 4 mL of blood were collected from coccygeal vessels in randomly selected cattle. Blood samples were centrifuged, and the serum was separated then stored at −20 °C until analysis. The serodiagnosis of T. gondii infection was tested using an indirect ELISA multispecies diagnosis kit (ID Screen, ID.VET. T. gondii-specific IgG antibody, Innovative Diagnostics, Montpellier, France) [1]. Following the instructions of the manufacturer, the test presented 98% sensitivity and 99% specificity. The ELISA kit was applied to detect IgG antibodies, using the P30 (SAG1) antigen and the multispecies HRP conjugate antibody as the secondary antibody [27]. TMB substrate was added, and the reaction was then measured at 450 nm. The results were interpreted from S/P% calculation, using this formula [(OD sample − ODNC)/(ODPC − ODNC)] × 100. Samples with S/P% less than 40% were considered negative, those ranging from 40 to 50% doubtful, and those exceeding 50% positive. The doubtful cases were collected for new sample after 2 weeks and retested.
Data collection
Data were obtained by interviewing and analyzed for risk factors associated with T. gondii seropositivity. This analysis included individual-level and herd-level analyses. The individual-level variables were as follows: dairy status (heifer/cow), parity, born in the farm (yes/no), abortion history during the past pregnancies (yes/no), and repeat breeding (yes/no). In addition, the herd-level variables included hospital member information (Kamphaeng Saen, KPS/Nong Pho, NP), location (Nakhon Pathom/Kanchanaburi/Ratchaburi), duration of farm, co-operative information (Nakhon Pathom, NKP/Sirichok, SR/Thamuang, TM/Nong Pho, NP), standard farm certification (yes/no), total number of cattle, farm type (tie stall/free-stall), presence of companion cats (yes/no), number of companion cats, presence of stray cats (yes/no), open stack of feed (yes/no), feeding with fresh grass (yes/no), pasture (yes/no), type of water (tap/ground/well), feeding area (ground/higher from the ground), frequency of cleaning per day, introduction of cattle from other farms (yes/no), and presence of soil or sand area in house (yes/no). These variables were used for interviewing farm owners.
Statistical analysis
The estimated true prevalence (TP) was calculated considering the apparent prevalence (AP), sensitivity (Se), and specificity (Sp) of the test, as follow [40].
A farm was considered positive if at least one animal from the farm was infected. All the studied variables were analyzed by univariate analyses (binary logistic regression) at both individual and farm levels. Subsequently, if the p value of each analyzed variable was equal to or less than 0.2, the variable was considered to have sufficient significance for building a logistic regression model. The mixed effects of the significant variables were built for the final model by logistic regression analysis [44]. The associations between variables and seropositive farms were interpreted using odds ratio with 95% CI both in univariate and logistic regression analyses. All analyses were performed using RStudio software, R version 4.0.5, 2021-03-31 [33].
Results
Toxoplasma gondii antibodies were detected in 22 out of the 901 serum samples; the apparent prevalence is 2.44% (95% CI, 1.55–3.399). The calculated true prevalence, derived from apparent prevalence, sensitivity and specificity of test, was 1.48% (95% CI, 0.64–2.75%). Seropositive rates across different parity ranged from 2.06 to 2.96%, showing closely similar percentages between each parity group. The univariate analysis between seropositive animal and factors presented no variables that cannot be included for logistic regression analysis. The summarized results are presented in Table 1.
Seropositivity percentage and univariate analysis results across individual level variables.
The results showed T. gondii seropositive farms were detected in all districts, there were 15 seropositive farms, seroprevalence at the farm-level was 29.41% (95% CI, 18.71–43%). In the univariate analysis, three variables were associated with T. gondii seropositivity, including the number of companion cats (OR = 1.12, p = 0.2), presence of stray cats (OR = 3.44, p = 0.067), and frequency of cleaning per day (OR = 3.37, p = 0.008). The average number of companion cats in the seropositive group was higher than the seronegative group. Additionally, the percentage of seropositivity was higher in the group with presence of stray cats than in the group without stray cats. Interestingly, the frequency of cleaning in one day was strongly significantly associated with T. gondii seropositivity. The results showed that seropositive farms had a higher frequency of cleaning per day compared to seronegative farms. The results of univariate analysis of farm-level seropositivity are presented in Table 2. Logistic regression analysis was performed using these three variables. Results showed that the frequency of cleaning per day was a potential risk factor related to T. gondii seropositive farms (OR = 2.745, 95% CI, 1.15–8.69, p = 0.02). These results are summarized in Table 3.
Seropositivity percentage and univariate analysis results across farm level variables.
Logistic regression analysis of farm level data.
Discussion
This study revealed a 1.48% prevalence of T. gondii infection in dairy cattle from the Western Region of Thailand. This prevalence was lower than that in previous reports from other parts of the country [4, 20–22]. Because the cattle in these previous reports included young animals (aged 0–1 year), this might be due to the effect of maternal immunity on their high seropositive results. Therefore, the lower T. gondii prevalence in this study may be influenced by the difference in the type of animal sample.
Previous studies in Thailand used the LAT for T. gondii antibody detection [4, 20–22]. Both IgG and IgM antibodies in serum were able to be agglutinated by parasite antigen in the LAT method. The IgM antibodies could be detected via nonspecific antigens from whole T. gondii lysates soluble in LAT. The conserved pathogen-associated molecular patterns (PAMPs) across species were close to those of T. gondii, which can react with IgM in serum. Therefore, this reaction could be a false-positive result for LAT [39]. In contrast, in the present study only IgG antibodies were detected, via the P30 (SAG1) antigen. Moreover, comparing the performance of the IDvet ELISA kit and the IFAT showed 82.48% sensitivity and 97.8% specificity [30]. The sensitivity and specificity of LAT when compared with those of the IFAT were 100% and 91.3%, respectively [45]. Because ELISA has a lower sensitivity and higher specificity than LAT, the percentage of positive results would be lower for ELISA. Thus, the low prevalence of T. gondii infection in this survey may be caused by the difference in the methods used for antibody detection.
The seroprevalence of T. gondii differs in various parts of the world, and this study found lower values than in other countries in South East Asia. The seroprevalence values were reported to be 2.59–6.3%, 7–9%, and 6.6% from Malaysia, Indonesia, and Myanmar, respectively [2, 6, 8, 31, 34, 37]. Reviews of T. gondii infections in South Asian cattle, including those from India, Pakistan, and Bangladesh, revealed seropositivity of 42% (CI, 31–49%), 25% (CI, 16–33%), and 12% (CI, 2.5–31%), respectively. The overall percentage of seropositivity from these countries was 27.9% [24]. All these countries surrounding Thailand conducted surveys in beef cattle, which are raised under different management practices compared to dairy cattle. Beef cattle typically roam in free-range pastures, whereas dairy cattle receive more intensive care. For example, a study from India showed that the seroprevalence of free-ranging mithuns (Bos frontalis) was 42%, which is higher than captive mithuns, 28% [35, 36]. China is the largest country in the region and has conducted extensive surveys on T. gondii in dairy cattle. Some reported seroprevalence rates were similar to those in our study, at 1.93% for the central part of China [12]. However, seroprevalence rates for other parts of China were higher, ranging from 4.87% to 13.71% [44, 46, 52]. This variation in T. gondii prevalence was dependent on diverse geographic and local climate conditions and environments.
Furthermore, South America has tropical climates and dairy production similar to Thailand. Previous studies have reported T. gondii seroprevalence in dairy cattle ranging from 8.48% to 32% [3, 18]. Also, herd prevalence ranged from 93% to 100%, which is higher than in this study [11, 18]. The weather and environment in Brazil are similar to those in Thailand, but the T. gondii seroprevalence is greater than that in Thailand. Because the Brazil dairy industry has a large intensive farming model and is combined with grazing pasture, the possibility of exposing infective oocysts in the environment is increased. Additionally, this area contains numerous wild felids that are in close contact with pasture and drinking water sources [11].
The individual factors of cattle including type, pregnancy status, cow status, and parity exhibited no relation with T. gondii infection. There was no difference in seroprevalence between young and old cattle. However, some reports indicated a significant increase in seroprevalence among young cattle; it also increased in older cattle [1, 17, 25, 46]. It is possible that young cattle may show greater susceptible to T. gondii infection, and their immune response is stronger than older cattle [16]. Furthermore, the high seroprevalence in older cattle may be attributed to prolonged exposure to T. gondii infection [1, 42]. In addition, owing to the results of this cross-sectional study, the association between age and antibody response should be monitored in the long-term in individual cattle.
Our study examined reproductive problems, including abortion history and repeat breeder cattle, but these results did not reveal an association between these two reproductive problems and positivity for T. gondii antibodies. Similarly, there was no significant relationship between infection with T. gondii in cattle in Brazil and abortion history, even though the prevalence of T. gondii was high [28]. Additionally, research in India and China showed that the prevalence of T. gondii infection in cattle was not correlated with abortion [19, 44]. Even though infected cattle appear to be resistant to toxoplasmosis, a study in Iran showed that the percentage of T. gondii-positive cattle with a history of abortion was significantly greater than that of cattle without a history of abortion [15]. Therefore, the severity of toxoplasmosis in cattle should be investigated to determine the virulence of protozoa in circulation from different areas.
The percentages of seropositive farms in Nakhonpathom, Kanchanaburi, and Ratchaburi Provinces were 20%, 40%, and 40%, respectively. There were positive farms identified from all observed districts, indicating widespread exposure of dairy cattle to T. gondii. The services provided by hospitals and cooperatives, as well as standard farm management practices, did not differ significantly in their association with T. gondii infection. Because cattle are infected via digestion of the sporulated oocyst of T. gondii, previous studies have shown that companion cats and stray cats are risk factors for T. gondii infection in dairy cattle [28, 41]. Our univariate analysis revealed factors for which the p value was equal to or less than 0.2, including the number of companion cats and the presence of stray cats. However, the multivariate analysis of these two factors revealed no significant associations with T. gondii-positive farms. Additionally, the likelihood ratio test of the presence of the stray cat factor was closely related. A report from Midwestern Brazil showed that T. gondii infection in beef cattle was not associated with the presence of cats on farms, but wild felids were the most important key to transmitting protozoa [28]. Briefly, the dairy cattle in Thailand were raised in a smallholder production system with a basic level of biosecurity, and companion and stray cats were allowed free contact with the house, feed, and drinking water of the cattle. This free-roaming behavior of cats increases the possibility of T. gondii infection via ingestion of sporulated oocysts or tissue cysts from intermediate hosts [50]. In addition, the prevalence of T. gondii infection in stray cats was greater than that in companion cats, suggesting that the risk posed by stray cats could lead to contamination of feed and water for dairy cattle or transmission to companion cats through oocysts [28, 29]. Thus, stray cats might be one of the factors influencing T. gondii circulation in this area.
Interestingly, the frequency of cleaning per day was associated with the number of T. gondii seropositive farms (OR = 2.74; 95% CI, 1.15–8.69; p = 0.043). Farmers usually clean the floor before milking twice a day. The cleaning protocol involved using high-pressure water and sweeping to remove feces. This study revealed a high frequency of water used for cleaning associated with T. gondii seropositive farms. Due to the prolonged environmental survivability of oocysts, coupled with cleaning procedures, their dissemination across the farm is facilitated. This might be the cause of the increased chance of cattle being infected with T. gondii. In addition, it is generally known that felines can shed more than 100 million oocysts per cat [38]. A previous study demonstrated that severity of toxoplasmosis in cattle depends on the inoculation dose of oocyst [14]. The infective dose of T. gondii that induced antibody development in cattle included 1.0 × 104–106 oocysts [7, 9, 13]. Thus, it might be that a small number of cats were able to produce enough oocyst to contaminate the environment, sufficiently to maintain disease circulation [19]. The authors consider, even though cats were not associated with T. gondii seropositivity, that the frequency of cleaning might increase the possibility of oocyst exposure in dairy cattle and increase T. gondii seropositive.
Conclusions
Toxoplasma gondii infection occurred with low seroprevalence which indicated that T. gondii continued to circulate in the dairy cow population in the western part of Thailand. This information provided further understanding of farm management systems and T. gondii infections on farms. The significant risk factor presented here was the high frequency of cleaning per day that supported the spread and maintenance of infection by T. gondii. Although cats were the definitive host of this parasite, they were not identified as a significant risk factor for infection. Moreover, it was recommended to conduct long-term observations of seroprevalence to visualize the dynamic of antibodies in cattle. Additionally, to enhance understanding of the epidemiology of T. gondii, similar studies should be carried out in other regions of Thailand.
Acknowledgments
This study was supported by joint funding from the Royal Golden Jubilee, PhD Program, under the Thailand Research Fund and Kasetsart University (Grant No. NRCT5-RGJ63002-034). The authors would like to thank veterinary research and development centers and farmers for supporting sample collection.
Conflicts of interest
The authors also declare that they do not have any conflicts of interest.
References
- Abdallah MC, Kamel M, Karima B, Samir A, Djamel K, Rachid K, Khatima AO. 2019. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep, and goats in Algeria: seroprevalence and risk factors. Veterinary Sciences, 6, 63. [CrossRef] [PubMed] [Google Scholar]
- Abdul Hamid N, Sadiq MB, Ramanoon SZ, Manor R, Watanabe M, Md Isa NM, Kamaludeen J, Syed-Hussain SS. 2020. Seroprevalence and risk factors of Toxoplasma gondii in ruminant meats from wet markets in Klang valley and abattoirs in Selangor, Malaysia. Animals, 10, 1139. [CrossRef] [PubMed] [Google Scholar]
- Alves L, Lima J, Melo J, de Castro AM, Soares V, Rossi G, Teixeira W, Ferreira L, Cruz B, Felippelli G, Oliveira V, Brom P, Krawczak F, da Costa AJ, Lopes W. 2021. Spatial distribution of Toxoplasma gondii in cows and associated risk factors. Tropical Animal Health and Production, 53, 76. [CrossRef] [PubMed] [Google Scholar]
- Arunvipas P, Inpankaew T, Jittapalapong S. 2008. Risk factors for toxoplasmosis in dairy cows in western Thailand, in Proceedings of the 46th Kasetsart University Annual Conference, Kasetsart, 29 January-1 February, 2008. Subject: Animals & Veterinary Medicine: Bangkok, Thailand. p. 384–389. [Google Scholar]
- Baldursson S, Karanis P. 2011. Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Research, 45, 6603–6614. [CrossRef] [PubMed] [Google Scholar]
- Bawm S, Htun LL. 2015. Parasitic zoonoses in livestock and domestic animals of Myanmar and neighboring countries. Asian Journal of Animal and Veterinary Advances, 10, 740–751. [CrossRef] [Google Scholar]
- Burrells AA, Taroda A, Opsteegh M, Schares G, Benavides J, Dam-Deisz Bartley PM, Chianini F, Villena I, van der Giessen J, Innes EA, Katzer F. 2018. Detection and dissemination of Toxoplasma gondii in experimentally infected calves, a single test does not tell the whole story. Parasites & Vectors, 11, 45. [CrossRef] [PubMed] [Google Scholar]
- Chandrawathani P, Zanin CM, Premaalatha B, Adman M, Jamnah O, Khor SK, Khadijah S, Lai SZ, Shaik MA, Seah TC, Zatil SA. 2008. Seroprevalence of Toxoplasma gondii antibodies in pigs, goats, cattle, dogs and cats in Peninsular Malaysia. Tropical Biomedicine, 25, 257–258. [PubMed] [Google Scholar]
- Costa GH, da Costa AJ, Lopes WD, Bresciani KD, dos Santos TR, Esper CR, Santana AE. 2011. Toxoplasma gondii: infection natural congenital in cattle and an experimental inoculation of gestating cows with oocysts. Experimental Parasitology, 127, 277–281. [CrossRef] [PubMed] [Google Scholar]
- da Costa MA, Pinto-Ferreira F, de Almeida RPA, Martins FDC, Pires AL, Mareze M, Mitsuka-Bregano R, Freire RL, da Rocha Moreira RV, Borges JM, Navarro IT. 2020. Artisan fresh cheese from raw cow’s milk as a possible route of transmission in a Toxoplasmosis outbreak, in Brazil. Zoonoses and Public Health, 67, 122–129. [CrossRef] [PubMed] [Google Scholar]
- de Azevedo Filho PCG, Ribeiro-Andrade M, dos Santos JF, dos Reis AC, de Araujo Valenca SRF, Samico Fernandes EFT, Pinheiro Junior JW, Mota RA. 2020. Serological survey and risk factors for Toxoplasma gondii infection in cattle from Amazonas, Brazil. Preventive Veterinary Medicine, 176, 104885. [CrossRef] [PubMed] [Google Scholar]
- Dong H, Lu YY, Su RJ, Wang YH, Wang MY, Jiang YB, Yang YR. 2018. Low prevalence of antibodies against Toxoplasma gondii in dairy cattle from China’s Central Region. BMC Veterinary Research, 14, 315. [CrossRef] [PubMed] [Google Scholar]
- Dubey JP, Thulliez P. 1993. Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. American Journal of Veterinary Research, 54, 270–273. [PubMed] [Google Scholar]
- Esteban-Redondo I, Maley SW, Thomson K, Nicoll S, Wright S, Buxton D, Innes EA. 1999. Detection of T. gondii in tissues of sheep and cattle following oral infection. Veterinary Parasitology, 86, 155–171. [CrossRef] [PubMed] [Google Scholar]
- Gharekhani J, Yakhchali M. 2020. Risk factors associated to Toxoplasma gondii infection in dairy farms in Hamedan Suburb, Iran. Journal of Parasitic Diseases, 44, 116–121. [CrossRef] [PubMed] [Google Scholar]
- Gilot-Fromont E, Aubert D, Belkilani S, Hermitte P, Gibout O, Geers R, Villena I. 2009. Landscape, herd management and within-herd seroprevalence of Toxoplasma gondii in beef cattle ferds from Champagne-Ardenne, France. Veterinary Parasitology, 161, 36–40. [CrossRef] [PubMed] [Google Scholar]
- Gomes DFC, Mendes LA, Dias JM, Ribeiiro-Andrade M, Oliveira PRF, Mota RA, Arnhold E, Fioarvanti MCS, Oliveira CHS. 2021. Seroprevalence, spatial distribution and risk factors associated with Toxoplasma gondii infection among cattle in a Quilombola Community in the Brazilian Cerrado. Revista Brasileira de Parasitologia Veterinaria, 30, e018720. [CrossRef] [PubMed] [Google Scholar]
- Guimaraes AM, Bruhn FRP, da Rocha C, de Araujo TH, Mesquita CAM. 2020. Seroepidemiology of Toxoplasma gondii in dairy cows in southeastern Brazil: seropositive cows on all farms investigated. Acta Parasitologica, 65, 628–635. [CrossRef] [PubMed] [Google Scholar]
- Hebbar BK, Mitra P, Khan W, Chaudhari S, Shinde S, Deshmukh AS. 2022. Seroprevalence and associated risk factors of Toxoplasma gondii and Neospora caninum infections in cattle in central India. Parasitology International, 87, 102514. [CrossRef] [PubMed] [Google Scholar]
- Inpankaew T, Pinyopanuwat N, Chimnoi W, Kengradomkit C, Sununta C, Zhang G, Nishikawa Y, Igarashi I, Xuan X, Jittapalapong S. 2010. Serodiagnosis of Toxoplasma gondii infection in dairy cows in Thailand. Transboundary and Emerging Disease, 57, 42–45. [CrossRef] [Google Scholar]
- Jittapalapong S, Sanwaranond A, Inpunkaew T, Phasuk T, Pinyopanuwat N, Chimnoi W, Kengradomkig C, Arunvipat P, Maruyama S. 2008. Seroprevalence of Toxoplasma gondii infection in dairy cows in Northeastern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health, 39, 1. [Google Scholar]
- Jittapalapong S, Pinyopanuwat N, Chimnoi W, Kengradomkij C, Arunvipas P, Sarataphan N, Maruyama S, Desquesnes. 2008. Seroprevalence of Brucella abortus, Neospara caninum, and Toxoplasma gondii infections of dairy cows in South of Thailand, in Proceedings of the 15th Congress of FAVA, FAVA-OIE Joint Symposium on Emerging Diseases, 27–30 October, Bangkok, Thailand. p. 271–272. [Google Scholar]
- Jones JL, Dubey JP. 2021. Foodborne toxoplasmosis. Clinical Infectious Diseases, 55, 845–851. [Google Scholar]
- Khan MU, Rashid I, Akbar H, Islam S, Riaz F, Nabi H, Ashraf K, Singla LD. 2017. Seroprevalence of Toxoplasma gondii in South Asian countries. Revue Scientifique et Technique, 36, 981–996. [CrossRef] [PubMed] [Google Scholar]
- Liu F, Wang D, Yang SC, Zhu JH, Li JM, Shi K, Du R, Zhao Q. 2019. Prevalence and risk factors of brucellosis, toxoplasmosis, and neosporosis among Yanbian Yellow cattle in Jilin Province, China. Vector Borne Zoonotic Diseases, 19, 217–221. [CrossRef] [PubMed] [Google Scholar]
- Liu Q, Wang ZD, Huang SY, Zhu XQ. 2015. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites & Vectors, 8, 292. [CrossRef] [PubMed] [Google Scholar]
- Liyanage K, Wiethoelter A, Hufschmid J, Jabbar A. 2021. Descriptive comparison of ELISAs for the detection of Toxoplasma gondii antibodies in animals: a systematic review. Pathogens, 10, 605. [CrossRef] [PubMed] [Google Scholar]
- Maia MO, de Almeida SLH, Schmidt AC, de Oliveira ACS, de Aguiar DM, dos Santos-Doni TR, de Campos Pacheco R. 2021. High prevalence of anti-Toxoplasma gondii antibodies in beef cattle in Midwestern Brazil. Veterinary Research Communication, 45, 399–407. [CrossRef] [PubMed] [Google Scholar]
- Majid A, Ahmad N, Haleem S, Akbar NU, Zareen S, Taib M, Khan S, Hussain R, Sohail. 2021. Detection of toxoplasmosis in pets and stray cats through molecular and serological techniques in Khyber Pakhtunkhwa, Pakistan. BMC Veterinary Research, 17, 357. [CrossRef] [PubMed] [Google Scholar]
- Mangili P, Vesco G, Feliziani F, Paloni A, Menichelli M, Cagiola M, Marini C, Pourquier P, Papa P. 2009. Development and evaluation of the performance of an in-house ELISA to be used for the indirect diagnosis of toxoplasmosis in sheep, in Proceeding of SIDILV, Parma, Italy. Poster 1–2. [Google Scholar]
- Matsuo K, Husin D. 1996. A survey of Toxoplasma gondii antibodies in goats and cttle in Lampung Province, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health, 27, 554–555. [Google Scholar]
- Opsteegh M, Spano F, Aubert D, Belea A, Burrells A, Cherchi S, Corelissen J, Dam-Deisz C, Guitian J, Gyorke A, Innes EA, Katzer F, Limon G, Possenti A, Pozio E, Schares G, Villena I, Wisselink HJ, van der Giessen JWB. 2019. The relationship between the presence of antibodies and direct detection of Toxoplasma gondii in slaughtered calves and cattle in four European Countries. International Journal of Parasitology, 49, 515–522. [CrossRef] [Google Scholar]
- R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Rahman W, Manimegalai V, Chandrawathani P, Narulaini R, Zaini C, Premaalatha B. 2011. Seroprevalence of Toxoplasma gondii in Malaysian cattle. Malaysian Journal of Veterinary Research, 2, 51–56. [Google Scholar]
- Rajkhowa S, Sarma DK, Rajkhowa C. 2006. Seroprevalence of Toxoplasma gondii antibodies in captive mithuns (Bos frontalis) from India. Veterinary Parasitology, 135, 369–374. [CrossRef] [PubMed] [Google Scholar]
- Rajkhowa S, Rajkhowa C, Chamuah J. 2008. Seroprevalence of Toxoplasma gondii antibodies in free-ranging mithuns (Bos frontalis) from India. Zoonoses and Public Health, 55, 320–322. [CrossRef] [PubMed] [Google Scholar]
- Retmanasari A, Widartono BS, Wijayanti MA, Artama WT. 2017. Prevalence and risk factors for toxoplasmosis in Middle Java, Indonesia. Ecohealth, 14, 162–170. [Google Scholar]
- Robert-Gangneux F, Darde ML. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 25, 264–296. [CrossRef] [PubMed] [Google Scholar]
- Sarfraz-Ur-Rahman, Akbar H, Shabbir MZ, Ullah U, Rashid MI. 2022. Serological investigation of bovine toxoplasmosis using commercial and indigenous ELISA kits while validating cattle Toxo Igg ELISA Kit. Animals, 12, 16. [Google Scholar]
- Sergeant ESG. 2018. Epitools epidemiological calculators. Ausvet. Available at: http://epitools.ausvet.com.au. [Google Scholar]
- Silva BM, Queiroz WCC, Maia MO, Pacheco RC, Aguiar DM, Campos MS, Bresciani KDS, Costa AJ, Gomes AAD, Santos-Doni TR. 2021. Seroprevalence and risk factors of Toxoplasma gondii in cattle from Unai, Minas Gerais State, Brazil. Veterinary Parasitology, Regional Studies and Reports, 25, 100610. [CrossRef] [PubMed] [Google Scholar]
- Sroka J, Karamon J, Wojcik-Fatla A, Piotrowska W, Dutkiewicz J, Bilska-Zajac E, Zajac V, Kochanowski M, Dabrowska J, Cencek T. 2020. Toxoplasma gondii infection in slaughtered pigs and cattle in Poland: seroprevalence, molecular detection and characterization of parasites in meat. Parasites & Vectors, 13, 223. [CrossRef] [PubMed] [Google Scholar]
- Stear MJ. 2005. OIE manual of diagnostic tests and vaccines for terrestrial animals (Mammals, Birds and Bees) 5th Edn. Volumes 1 & 2. World Organization for Animal Health 2004. Parasitology, 130(6), 727–727. [Google Scholar]
- Sun WW, Meng QF, Cong W, Shan XF, Wang CF, Qian AD. 2015. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhea virus in commercial dairy and beef cattle in Eastern, Northern and Northeastern China. Parasitology Research, 114, 4211–4218. [CrossRef] [PubMed] [Google Scholar]
- Sunanta C, Inpankaew T, Pinyopanuwat N, Chimnoi W, Kengradomkij C, Arunvipas P, Maruyama W, Jitapalapong S. 2009. Comparison of diagnostic technique for detection of Toxoplasma gondii infection in dairy cows in Thailand. Agriculture and Natural Resources, 43, 48–52. [Google Scholar]
- Tan QD, Yang XY, Yin MY, Hu LY, Qin SY, Wang JL, Zhou DH, Zhu XQ. 2015. Seroprevalence and correlates of Toxoplasma gondii infection in dairy cattle in Northwest China. Acta Parasitologica, 60, 618–621. [PubMed] [Google Scholar]
- Thrusfield M, Christley R, Brown H, Diggle PJ, French N, Howe K, Kelly L, O’Connor A, Sargeant J, Wood H. 2018. Veterinary epidemiology. John Wiley & Sons, Hoboken, New Jersey, USA. [CrossRef] [Google Scholar]
- Tilahun B, Tolossa YH, Tilahun G, Ashernafi H, Shimelis S. 2018. Seroprevalence and risk factors of Toxoplasma gondii infection among domestic ruminants in East Hararghe Zone of Oromia Region, Ethiopia. Veterinary Medicine International, 2018, 4263470. [CrossRef] [Google Scholar]
- Torrey EF, Yolken RH. 2013. Toxoplasma oocytes as a public health problems. Trends in Parasitology, 29, 380–384. [CrossRef] [PubMed] [Google Scholar]
- Udonsom R, Buddhirongawatr R, Nishikawa Y, Fereig RM, Jirapattharasate C. 2021. Toxoplasma gondii prevalence and risk factors in owned domestic cats from Nakhon Pathom Province, Thailand. Veterinary Integrative Sciences, 19, 557–566. [CrossRef] [Google Scholar]
- Wiengcharoen J, Thompson RC, Nakthong C, Ratanakorn P, Sukthana Y. 2011. Transplacental transmission in cattle: is Toxoplasma gondii less potent than Neospora caninum? Parasitology Research, 108, 1235–1241. [CrossRef] [PubMed] [Google Scholar]
- Xu MJ, Liu QY, Fu JH, Nisbet AJ, Shi DS, He XH, Pan Y, Zhou DH, Song HQ, Zhu XQ. 2012. Seroprevalence of Toxoplasma gondii and Neospora caninum infection in dairy cows in subtropical southern China. Parasitology, 139, 1425–1428. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Wannapong N, Lertwatcharasarakul P & Rukkwamsuk T. 2024. Prevalence and risk factors of Toxoplasma gondii infection in dairy cattle from the Western Region of Thailand. Parasite 31, 38.
All Tables
Seropositivity percentage and univariate analysis results across individual level variables.
Seropositivity percentage and univariate analysis results across farm level variables.
All Figures
 |
Figure 1 Study area in three provinces in the western part of Thailand. Geographical distribution of the dairy farms in this area, including T. gondii seropositive farms (red circles) and negative farms (green circles). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



![$$ n=\enspace \frac{\sum_{i=1}^e[\frac{{\enspace \left({n}_i\right)}^2\times {p}_i\times \left(1-{p}_{i\enspace }\right)}{{W}_i}\enspace ]}{{N}^{2\enspace }\times \frac{{{AE}}^2}{{Z}^2}+\sum_{i=1}^e[{n}_{i\enspace }\times \left(1-{p}_{i\enspace }\right)]} $$](/articles/parasite/full_html/2024/01/parasite240026/parasite240026-eq1.gif)
![$$ n=[1-{\left(1-{p}_1\right)}^{\frac{1}{d}\enspace }]\enspace [\mathrm{N}-\mathrm{d}/2]\enspace +\enspace 1 $$](/articles/parasite/full_html/2024/01/parasite240026/parasite240026-eq2.gif)