| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 26 | |
| Number of page(s) | 25 | |
| DOI | https://doi.org/10.1051/parasite/2024024 | |
| Published online | 22 May 2024 | |
urn:lsid:zoobank.org:pub:883B4851-DF29-422E-A27F-69CF8987551D
Research Article
Untangling the Derogenes varicus species complex in Scandinavian waters and the Arctic: description of Derogenes abba n. sp. (Trematoda, Derogenidae) from Hippoglossoides platessoides and new host records for D. varicus (Müller, 1784) sensu stricto
Démêler le complexe d’espèces Derogenes varicus dans les eaux scandinaves et arctiques : description de Derogenes abba n. sp. (Trematoda, Derogenidae) parasite d’Hippoglossoides platessoides et nouveaux signalements d’hôtes pour D. varicus (Müller, 1784) sensu stricto
1
Department of Zoology, Swedish Museum of Natural History, Box 50007, SE-104 05, Stockholm, Sweden
2
Australian National Insect Collection, National Research Collections Australia, CSIRO, PO Box 1700, Canberra, ACT 2601, Australia
3
Department of Biological Sciences, University of Bergen, PO Box 7803, N-5020 Bergen, Norway
4
Department of Evolution, Ecology and Behaviour, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, L69 7AB, UK
* Corresponding author: chahinezbouguerche@gmail.com
Received:
2
January
2024
Accepted:
25
April
2024
Several studies have shown that the euryxenic trematode Derogenes varicus (Müller, 1784) represents a species complex. Four lineages have been designated (DV1–4) with the DV1 clade corresponding to D. varicus sensu stricto. Herein, we investigate newly collected specimens of D. varicus sensu lato from Scandinavian and Arctic waters using integrative taxonomy. The trematodes were collected from Melanogrammus aeglefinus, Eutrigla gurnardus, Trachinus draco, and Merluccius merluccius off the Atlantic coast of Sweden and from Hippoglossoides platessoides from Arctic Svalbard. 28S sequences of derogenids from Sweden were identical to D. varicus sensu stricto, confirming its euryxeny. The 28S sequences of Derogenes sp. from H. platessoides were identical to Derogenes DV2 and differed from D. varicus sensu stricto by 3% and from Derogenes DV3 by 2%. The 28S sequence divergences of Derogenes sp. from H. platessoides with D. ruber and D. lacustris were 3 and 10%, respectively. ITS2 and cox1 divergences between Derogenes sp. from H. platessoides and other Derogenes species/lineages were at levels of interspecific differences. The species from H. platessoides is described here as D. abba n. sp. We also examined the type material of Progonus muelleri (Levinsen, 1881), the type and only species of the genus Progonus, with redescription and designations of paralectotypes. Based on specimens from Theodor Odhner’s collections at the Swedish Museum of Natural History, SMNH, Stockholm, we provide novel morphological and anatomical data for D. varicus sensu lato species complex. Lastly, we investigated Arthur Looss’s “lost collection” of Trematodes at the SMNH and characterised a putative species Derogenes sp. “limula”.
Résumé
Plusieurs études ont montré que le trématode euryxene Derogenes varicus (Müller, 1784) représente un complexe d’espèces. Quatre lignées ont été désignées (DV1–4), le clade DV1 correspondant à D. varicus sensu stricto. Ici, nous étudions des spécimens nouvellement collectés de D. varicus sensu lato dans les eaux scandinaves et arctiques en utilisant la taxonomie intégrative. Les trématodes ont été collectés de Melanogrammus aeglefinus, Eutrigla gurnardus, Trachinus draco et Merluccius merluccius au large de la côte atlantique de la Suède et d’Hippoglossoides platessoides du Svalbard arctique. Les séquences 28S des Derogenidae de Suède étaient identiques à D. varicus sensu stricto, confirmant son euryxénie. Les séquences 28S de Derogenes sp. de H. platessoides étaient identiques à Derogenes DV2 et différaient de D. varicus sensu stricto par 3% et de Derogenes DV3 par 2%. Les divergences des séquence 28S de Derogenes sp. de H. platessoides avec D. ruber et D. lacustris étaient respectivement de 3 et 10%. Les divergences ITS2 et cox1 entre Derogenes sp. de H. platessoides et d’autres espèces/lignées de Derogenes se situaient à des niveaux de différences interspécifiques. L’espèce de H. platessoides est décrite ici comme Derogenes abba n. sp. Nous avons également examiné le matériel type de Progonus muelleri (Levinsen, 1881), type et seule espèce du genre Progonus, avec une redescription et des désignations de paralectotypes. Sur la base de spécimens des collections de Theodor Odhner au Musée suédois d’histoire naturelle (SMNH), Stockholm, nous fournissons de nouvelles données morphologiques et anatomiques sur le complexe d’espèces de D. varicus sensu lato. Enfin, nous avons étudié la « collection perdue » de Trématodes d’Arthur Looss au SMNH et caractérisé une espèce putative, Derogenes sp. « limula ».
Key words: Derogenes varicus / Progonus muelleri / cryptic species / cox1 / Norway / Sweden
© C. Bouguerche et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A common problem in taxonomy and biodiversity assessment is the recognition of cryptic species. Although morphologically indistinguishable, cryptic species are genetically divergent, which may lead to unclear species boundaries between taxa. Additionally, the identification and study of cryptic species, along with the classification of organisms into nominal species, has importance beyond mere biodiversity assessment. This is particularly significant in the context of helminth parasites that impact human and veterinary health, as the presence of cryptic species can influence important medical and epidemiological factors such as pathogenicity and drug resistance [11].
The frequency of cryptic species varies among helminth groups [11]. Within the Trematoda, the richest known fauna of all the major metazoan taxa of fishes [6], cryptic species are frequently recognised with reports of them from at least 20 families [8]. Previous solid studies have demonstrated that encountering cryptic trematode species has become so frequent that their presence can almost be anticipated and should definitely be considered [12, 16]. In trematodes, firstly, there is the issue of phenotypic variation, which can mask common characteristics, potentially hindering identification. Secondly, there is the challenge of cryptic species, where species may appear similar externally but are genetically distinct [12]. Trematode species with similar morphology, but reported from wide host and geographic ranges, are often revealed as complexes with cryptic species [21]. Molecular techniques are indispensable for identifying cryptic species, as demonstrated in various trematode families [12]. The records of the derogenid Derogenes varicus (Müller, 1784), from 69 fish species across 33 families worldwide [3], displaying an exceptional euryxeny, hint that cryptic species may be present, and it has frequently been suggested that this species represents a species complex [19, 28]. Krupenko et al. [30] used molecular evidence to recognise four genetic lineages and infer that it is a species complex (designated as “DV1–4”). By analysing 28S sequences of D. varicus from the type-host, the Atlantic salmon Salmo salar from Norway, Bouguerche et al. [3] demonstrated that the DV1 clade is D. varicus sensu stricto (s. s.).
Herein, we investigate specimens of D. varicus sensu lato (s. l.) collected from several unrelated marine fish species, from Scandinavian and Arctic waters.
Based on DNA sequences of the second internal transcribed spacer (ITS2), the large subunit ribosomal DNA (28S) and the subunit I (cox1) in mtDNA and the examination of newly collected specimens, we provide a formal description of a new species in the genus Derogenes Lühe, 1900, recognised and described from the American plaice Hippoglossoides platessoides. We also identify D. varicus s. s. using 28S DNA sequences from additional host species. Examination of additional specimens of D. varicus s. l. from Sweden and Norway, Northeast Atlantic, from Theodor Odhner’s collections at the Swedish Museum of Natural History (SMNH), Stockholm, enabled us to provide novel morphological and anatomical data for D. varicus s. l. from various hosts. We also examined the type material of Progonus muelleri (Levinsen, 1881), the type and only species of the genus Progonus Looss, 1899 from the shorthorn sculpin Myoxocephalus scorpius from Aasiaat [Egedesminde], Greenland. Finally, we further investigated Arthur Looss’s “lost collection” of trematodes, bought by T. Odhner and preserved at the SMNH and we characterise a putative species Derogenes sp., an unpublished species referred to by A. Looss as “Derogenes limula”. The importance of integrative taxonomy is stressed here and demonstrated for species delimitations within the D. varicus species complex and in the genus Derogenes.
Material and methods
Host and parasite collection
Fishes were collected from off Sweden, Northeast Atlantic (Skagerrak, Kattegat, and Gullmarsfjorden) and off Svalbard, Arctic Norway (Table 1).
Fishes examined from Scandinavian waters of the North Sea, Northeast Atlantic and the Arctic Ocean during this study.
Specimens from Skagerrak and Kattegat were collected by the SLU Aqua team as part of the biannual International Bottom Trawl Survey, within the scope of their research projects and permits. Specimens from Gullmarsfjorden were collected in the vicinity of Kristineberg Center for Marine Research and Innovation, outside of the borders of the Gullmarns nature reserve, and within the scope of the permit for animal research from the Swedish Board of Agriculture (Enheten för försöksdjur och sällskapsdjur, Jordbruksverket, Dnr. 5.2.18-5483/18) and ethical approval for animal research from the Uppsala animal ethics committee (Uppsala djurförsöksetiska nämnd, Jordbruksverket, Dnr. 5.8.18-17209/2021) issued to the Swedish Museum of Natural History. Specimens from the Arctic Ocean were collected during the HHUMTL22 cruise by the Arctic University Museum of Norway, within the scope of the fieldwork sampling permit issued by the governor of Svalbard (RiS-1D12021Al) and the permission to trawl from the Norwegian Directorate of Fisheries (21/16250). Fishes were euthanised and made available for examination.
Several specimens of greater weever Trachinus draco, grey gurnard Eutrigla gurnardus, European hake Merluccius merluccius, and haddock Melanogrammus aeglefinus from Skagerrak and Kattegat were collected during the biannual International Bottom Trawl Survey by the SLU along the Swedish coast (Table 1). Eight specimens of M. aeglefinus from Gullmarsfjorden were collected in the vicinity of the Kristineberg Center for Marine Research and Innovation.
The American plaice H. platessoides from the Arctic Ocean was collected by bottom trawl, at 79 59.860558 N, 15 27.879328 E, and 170 m depth [68].
Digeneans were collected from freshly killed fishes. The gastrointestinal tract was removed and examined for trematodes using the gut wash method [14, 26]. Digeneans were fixed in near-boiled saline without pressure and preserved immediately in 80% ethanol for morphological and molecular studies. Nine specimens were processed as hologenophores (sensu Pleijel et al. [53]). Type specimens of P. muelleri were requested from the Natural History Museum of Denmark (SNM) and nine specimens of P. muelleri from M. scorpius from Aasiaat [Egedesminde] Greenland preserved in 70% ethanol and marked as holotype (old catalogue number ZMUC-TRE-000032) were received. Two of the specimens were stained in acetocarmine and studied by microscopy.
Additional studied specimens include derogenids in the invertebrate collections at the Swedish Museum of Natural History (SMNH): 1. D. varicus s. l. from the Northeast Atlantic from T. Odhner’s collections, ex the European flounder Platichthys flesus from Gullmarsfjorden, Kristineberg, Sweden, the argentina Argentina sphyraena, the common ling Molva molva, the cusk Brosme brosme, and the Atlantic halibut Hippoglossus hippoglossus from Trondheim, Norway; 2. P. muelleri ex M. scorpius from Spitsbergen, Svalbard, Arctic Norway; 3. Derogenes sp. ex the tentacled blenny Parablennius tentacularis from Trieste, Italy, Central Mediterranean from A. Looss’s collection.
Morphological methods
Preserved specimens of Derogenes including hologenophores were stained in iron acetocarmine, destained with acid-alcohol (1% HCl in 70% ethanol), dehydrated in an ethanol series (70–100%), cleared in clove oil, and mounted in Canada balsam. Two specimens of the type material of P. muelleri were stained according to the same methods and mounted on two separate slides (NHMD-114950).
Drawings were made through a Nikon Eclipse i80 microscope with DIC (differential interference contrast) and a drawing tube. Drawings were scanned and redrawn on a computer with Adobe Illustrator 2023. Stained specimens were measured by ImageJ ver. 1.53K [57]. Measurements are in micrometres and indicated as the range followed by the number of measurements in parentheses. Types and vouchers were deposited at the Swedish Museum of Natural History (SMNH), Stockholm, Sweden; the Natural History Museum of Denmark (SNM), Copenhagen, Denmark and the Arctic University Museum of Norway (UiT), Tromsø, Norway.
Molecular methods
Genomic DNA was extracted from seven hologenophores of D. varicus s. l. from T. draco, E. gurnardus, M. merluccius, M. aeglefinus, and two hologenophores of Derogenes sp. from H. platessoides. Genetic data were generated for three markers: cox1, ITS2, and the 28S rDNA. Small fragments of each hologenophore (posterior third) were placed in a 1.5 mL microcentrifuge tube containing 20 μL buffer ATL (QIAGEN, Hilden, Germany). For extraction of genomic DNA (gDNA), 20 μL buffer ATL and 20 μL proteinase K were added to each sample, followed by vortexing and incubation in an incubating microplate shaker at 56 °C and 300 rpm overnight. The lysed samples were processed to obtain gDNA, following the manufacturer’s instructions for gDNA extraction using a QIAGEN QiAmp DNA Microkit. The PCR reaction was performed following Bouguerche et al. [3].
The cox1 was amplified with the primers and cycling profile used by Krupenko et al. [30]. Primers, amplification, and sequencing protocols for the 28S rDNA regions followed Pérez-Ponce de León and Nadler [52] and García-Varela and Nadler [17]. ITS2 sequences were amplified using the primers 3S [45] and ITS2.2 [13]. PCR products were purified by Ampure XP Kit (Beckman Coulter Inc., Brea, CA, USA) and sequenced in both directions on a 3730 l DNA Analyser 96-capillary sequencer (Applied Biosystems, Waltham, MA, USA). We used CodonCode Aligner 3.7.1 (Codon Code Corporation, Centerville, MA, USA) to edit sequences, compared them to the GenBank database content with BLAST, and deposited them in GenBank under accession numbers PP314018, PP314019, PP314020, PP314022, PP384389, and PP384390.
Trees and distances
Phylogenetic analyses were performed using the newly generated sequences of Derogenes spp. and those of related species available in GenBank (Table 2), mainly the D. varicus complex and P. muelleri complex provided by Krupenko et al. [30] and by Bouguerche et al. [3]. Alignments for each gene region were constructed separately in AliView [32], then trimmed to the shortest sequence. Phylogenetic tree inference was carried out by the maximum likelihood (ML) method using MEGA11 [66]. Nucleotide substitution models for phylogenetic analyses using the ML method were selected using MEGA11 [66]. The Hasegawa-Kishino-Yano with Gamma Distributed (HKY+ G) model [23] was selected for the 28S, the Kimura 2-parameter (K2) model [27] for ITS2, and the Tamura-Nei model with Gamma Distributed with Invariant sites (TN93+ G+I) [65] for cox1. The probabilities were computed by the bootstrap analysis of 500 replications. We also constructed phylogenetic trees of respective regions for the same data sets using the neighbour-joining (NJ) method [56] with MEGA11, with 2,000 bootstraps computed for cox1, ITS2, and 28S. The p-distances [27] were computed from the same datasets with MEGA11.
Results
Morphology
Family Derogenidae Nicoll, 1910
Subfamily Derogeninae Nicoll, 1910
Genus Derogenes Lühe, 1900
Derogenes abba n. sp. (Figs. 1A–1C)
urn:lsid:zoobank.org:act:7CB281BD-05E0-4BAF-A717-A1E4E1477AE1
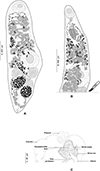 |
Figure 1 Derogenes abba n. sp. ex Hippoglossoides platessoides. A, whole body (Type-9562). B, terminal genitalia (Type-9562). C, hologenophore, forebody (Type-9563). |
Synonyms: Derogenes varicus DV2 of Krupenko et al. [30]; D. varicus of Olson et al. [50].
Type-host: Hippoglossoides platessoides (Pleuronectiformes: Pleuronectidae), American plaice.
Type-locality: Svalbard, Norway, Arctic Ocean, at 79 59.860558 N, 15 27.879328 E, and 170 m depth.
Other hosts: Invertebrate hosts, first intermediate hosts: Amauropsis islandica (Naticidae), Iceland moonsnail; Euspira pallida (Naticidae), Pale moonsnail; Buccinum scalariforme (Buccinidae), ladder whelk (definitive host).
Other localities: United Kingdom, North Sea [50]. Keret Archipelago, White Sea. Dalniye Zelentsy, Barents Sea [30].
Site in host: Stomach.
Deposited examined material: Holotype (SMNH- Type-9562), 11 paratypes (SMNH- Type-9563–9573), and 2 paratypes with molecular information (hologenophores) (SMNH- Type-9563–9564) deposited in the Type collections of the Swedish Museum of Natural History (SMNH), Stockholm, Sweden. One paratype (TSZY-519) deposited in the collections of The Arctic University Museum of Norway (UiT), Tromsø, Norway.
Paratypes with molecular information: anterior parts of specimens mounted on a slide, posterior part used for molecular analysis: slide SMNH- Type-9563; slide SMNH- Type-9564.
Representative DNA sequences: Partial 28S, two sequences (GenBank, PP314018–PP314019); ITS2, two sequences (GenBank, PP314020, PP314022); Partial cox1, two sequences (GenBank PP384389–PP384390).
Additional material examined for comparison: Whole mounts: (1) Eighteen specimens of D. varicus s. s. ex Salmo salar, Bremanger, Norway, Northeast Atlantic (SMNH 218683–218700). (2). Ten specimens of D. varicus s. s. ex Gadus morhua from Svalbard, Norway, Arctic (TSZY-520–529). (3). Derogenes varicus s. l. from the collection of T. Odhner in the Invertebrates collection of the Swedish Museum of Natural History (SMNH): one specimen ex Limanda limanda from Gullmarsfjorden, Kristineberg, Sweden, NEA (SMNH-114551); one specimen ex Argentina sphyraena from Trondheim, Norway, NEA (SMNH-114558); one specimen ex Molva molva Trondheim, Norway, NEA (SMNH-114559); one specimen ex Brosme brosme from Trondheim, Norway, NEA (SMNH-114560); three specimens ex Hippoglossus hippoglossus from Trondheim, Norway, NEA (SMNH-104577); five specimens ex Platichthys flesus from Gullmarsfjorden, Kristineberg, Sweden, NEA (SMNH-208360).
Etymology: Named after ABBA, the Swedish pop supergroup renowned for hits like “Dancing Queen”, “Chiquitita” and “Money, Money, Money” which served as a source of entertainment for the first author during the creation of the illustrations. The group’s name is an acronym of the first letters of their first names arranged as a palindrome. Invariable, treated as a noun in apposition.
Description: Measurements and comparisons in Tables 3–5. Body stocky (Figs. 1A and 1B), nearly sausage-shaped; anterior and posterior ends rounded. Pre-oral lobe short. Oral sucker rounded. Pre-pharynx absent. Pharynx muscular. Oesophagus short. Intestines bifurcating anterior to sinus-organ. Intestinal caeca extending posteriorly to vitelline masses and terminating blindly. Ventral sucker rounded.
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Atlantic. *Diameter. 1Note that D. lacustris is a freshwater species.
Male terminal genitalia in forebody. Sinus-sac oval to pyriform (Figs. 1B and 1C). Cone-shaped permanent muscular sinus-organ projecting into genital atrium. Ejaculatory duct and metraterm projecting into sinus-organ. Hermaphroditic duct thin-walled. Pars prostatica relatively short, lined by gland cells, leading to seminal vesicle. Seminal vesicle voluminous, oval, and thin-walled, situated in mid-forebody at considerable distance from ventral sucker. Testes oval to globular, symmetrical, posterior to ventral sucker.
Ovary globular, voluminous, post-testicular, sometimes overlapped by right vitelline mass. Laurer’s canal not observed. Vitelline masses in hindbody, round to oval, paired, situated on each side of body. Vitelline ducts fuse antero-medial to ovary. Seminal receptacle not observed. Uterus convoluted, uterine coils extending from near posterior extremity to sinus sac. Eggs oval. Excretory vesicle Y-shaped; branches reuniting dorsal to pharynx.
The morphology of the cercaria was described by Krupenko et al. [30].
Derogenes sp. (unpublished Derogenes limula of Looss, see discussion) (Figs. 2–4)
Host: Parablennius tentacularis (Blenniiformes: Blenniidae), tentacled blenny.
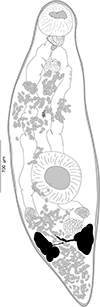 |
Figure 2 “Derogenes limula” ex Parablennius tentacularis (SMNH-208361). |
Locality: Trieste, Italy, Central Mediterranean [25].
Site in host: Intestine.
Material examined: One specimen from Parablennius tentacularis off Trieste, Italy (SMNH-208361), from the collection of A. Looss in the Invertebrates collection of the Swedish Museum of Natural History (SMNH), Stockholm, Sweden (identified and labeled by A. Looss as Derogenes sp.). One specimen from P. tentacularis off Trieste, Italy (SMNH-222309), stained from the wet collection of A. Looss (894) at the SMNH; identified and labeled by A. Looss as “Derogenes limula”.
Archival documents: in addition to a single slide mounted by A. Looss (SMNH-208361) (Fig. 2), the archives include two unpublished line drawings (Figs. 3A and 3B), combined and reproduced in Figures 4A–4E.
 |
Figure 3 “Derogenes limula” ex Parablennius tentacularis. Unpublished line drawing by A. Looss. |
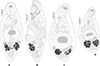 |
Figure 4 “Derogenes limula” ex Parablennius tentacularis based on A. Looss’s unpublished line drawings. A, Whole body, ventral view. B, Whole body, lateral view. C, Whole body, ventral view. D, Whole body, ventral view. |
Description: Measurements in Table 6. Body stocky, rounded anteriorly, pointed posteriorly (Figs. 4A, 4C, 4D), visibly larger at level of ventral sucker (Figs. 4C–4D). Tegument crenulate, with fine spines (Fig. 4B).
Oral sucker elongate, oval. Pharynx muscular, subglobular (Figs. 4A, 4C, 4D). Oesophagus short, caecal bifurcation at level of pharynx. Drüsenmagen absent (Fig. 4D). Caeca broad, extending to posterior region of hindbody, as far as uterus (Fig. 4B). Ventral sucker round to transversely oval, voluminous (Figs. 4A, 4C, 4D), larger than oral sucker.
Testes paired, oval, small, in hindbody, immediately posterior to ventral sucker (Fig. 4A). Ovary oval, voluminous, located between tested to slightly posteriorly to testes (Figs. 4A, 4C, 4D).
Vitellarium paired, in hindbody, posterior to testes; vitelline masses rosette-shaped, deeply lobed, connected one to another by a visible isthmus; composed both of 6–7 lobes (Figs. 4A, 4C, 4D). Uterine coils extending from posterior third of forebody to posterior end (Figs. 4A–4D), existing also in inter-vitelline masses space. Eggs numerous, large (Fig. 4A–4D). Excretory vesicle and position of bifurcation of stem not examined; excretory arms extending into forebody and unite dorsally to genital terminalia and dorsally to oral sucker.
Remarks: Derogenes sp. that we described above was found in A. Looss’s collection, labelled as “Derogenes limula”. As there are no published records of a species under that name, A. Looss probably intended to describe this Derogenes specimen from P. tentacularis as a new species, with the name “D. limula”. The eggs of this Derogenes sp. that we described above are over 40 μm and the species is thus consistent with “the large eggs group”. We compared the single specimen of Derogenes sp. (or “D. limula” as initially labeled by A. Looss) ex P. tentacularis to the Mediterranean congeneric Derogenes species. The present specimen Derogenes sp. ex P. tentacularis differs from D. minor, D. robustus, D. affine, and D. latus by its larger eggs. It resembles D. ruber in egg size (61 × 39 in Derogenes sp. vs. 62 × 39 in D. ruber) and in having lobed, tear-shaped vitelline masses. However, Derogenes sp. ex P. tentacularis can be readily distinguished from D. ruber by being smaller in all body measurements including the body (855 × 253 vs. 7869 × 1847). Derogenes sp. ex P. tentacularis can be easily distinguished from D. varicus s. s. and D. abba n. sp. by having lobed vitelline masses.
Progonus muelleri (Levinsen, 1881) (Figs. 5A and 5B)
Type-host: Myoxocephalus scorpius (as Cottus scorpius) (Perciformes: Cottidae), shorthorn sculpin [35].
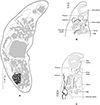 |
Figure 5 Progonus muelleri (Levinsen, 1881) ex Myoxocephalus scorpius (NHMD-114950) and comparison between the terminal genitalia in Progonus and in Derogenes. A, P. muelleri ex M. scorpius (NHMD-114950), whole body. B, P. muelleri ex M. scorpius, terminal genitalia, horizontal section (SMNH-114586). C, Derogenes varicus sensu stricto ex Limanda limanda, terminal genitalia, horizontal section (SMNH-114557). |
Other hosts: Gadus macrocephalus (as Gadus ovak) (Gadiformes: Gadidae), Greenland cod [35].
Type-locality: Aasiaat [Egedesminde], Greenland [35].
Site in host: Stomach.
Deposited examined material:
Whole mounts: Two specimens of P. muelleri ex Myoxocephalus scorpius from Aasiaat [Egedesminde], Greenland, deposited at the Natural History Museum of Denmark (SNM), Copenhagen, Denmark (NHMD-114950/ old catalogue number ZMUC-TRE-000032), Paralectotypes.
Transverse sections: T. Odhner’s collections in the Invertebrates collection of the Swedish Museum of Natural History (SMNH), Stockholm, Sweden; two specimens ex Myoxocephalus scorpius from Spitsbergen, Svalbard, Norway, Arctic (SMNH 114584–114585) (identified and labeled by T. Odhner as P. muelleri); One specimen from the same host and locality (SMNH 114587) (identified and labeled by T. Odhner as Genarches muelleri (Levinsen, 1881)).
Longitudinal sections: T. Odhner’s collections in the Invertebrates collection of the SMNH; one specimen ex Myoxocephalus scorpius from Spitsbergen, Svalbard, Norway, Arctic (SMNH 114586) (identified and labeled by T. Odhner as P. muelleri).
Additional material examined for comparison: Transverse sections: T. Odhner’s collections in the Invertebrates collection of the SMNH: four specimens of D. varicus s. s. ex Limanda limanda from Gullmarsfjorden, Kristineberg, Sweden, NEA (SMNH 114554–114557) (identified and labeled by T. Odhner as D. varicus).
Redescription: Body elongated, fusiform, rounded anteriorly (Fig. 5A). Tegument smooth, unarmed. Pre-oral lobe present. Oral sucker subterminal, small, rounded. Prepharynx not observed. Pharynx subglobular. Oesophagus not observed. Intestinal bifurcation in anterior third of forebody. Caeca wide, extending to and joining in anterior hindbody, forming cyclocoel, approximatively at level of testis. Ventral sucker subglobular, voluminous, clearly larger than oral sucker.
Genital pore a rounded pit, posterior to pharynx. Sinus sac oval to fusiform. Sinus-organ muscular, conical (Fig. 5B). Hermaphroditic duct thin walled. Seminal vesicle located in mid-forebody, saccular, fusiform. Pars prostatica short, lined with glandular cells. Testes two, oval, immediately posterior to ventral sucker.
Ovary globular, immediately post testicular. Uterus dorsal, extending to genital terminalia. Metraterm in midforebody, muscular. Eggs oval. Vitelline masses two, postovarian, sub-globular. Excretory pore posterior, terminal. Excretory vesicle not detected. Excretory branches joining in forebody, dorsally to pharynx.
Remarks: The type-material received from Natural History Museum of Denmark (SNM), Copenhagen, Denmark includes nine specimens preserved in 70% ethanol from M. scorpius off Aasiaat [Egedesminde], Greenland, and were indicated as the Holotype. Two specimens were stained in acetocarmine, mounted on slides, and designated herein as Paralectotypes. Comparative terminal genitalia of D. varicus s. s. shown in Figure 5C.
Molecular characterisation
The NJ and ML methods led to similar tree topologies and thus only the ML trees are shown (Figures 6–8).
 |
Figure 6 Tree inferred using the maximum likelihood method based on the 28S rDNA sequence data; only bootstrap values higher than 70 are indicated. The newly generated sequences of Derogenes varicus sensu stricto from Sweden, Northeast Atlantic are indicated by*. Other D. varicus sensu stricto are those of Krupenko et al. [30] from the Barents Sea and White Sea; and those of Bouguerche et al. [3] from Northeast Atlantic, off Sweden and Norway and from Svalbard, Norway, Arctic Ocean. |
The 28S dataset included 53 nucleotide sequences of the Derogenidae Nicoll, 1910. The trimmed matrix comprised 642 positions. The newly generated sequences of D. varicus ex M. aeglefinus, E. gurnardus, T. draco, and M. merluccius from Sweden were identical and clustered within the D. varicus s. s. clade without any host-related structuring.
The two newly generated 28S sequences for D. abba n. sp. ex H. platessoides were identical to each other and to sequences of rediae of D. varicus DV2 of Krupenko et al. [30] ex the gastropod intermediate mollusc hosts Euspira pallida and Amauropsis islandica from the White Sea and the Barents Sea; they were also identical to adults ex B. scalariforme from the White Sea and from the definitive host H. platessoides from the North Sea. All sequences divergence of D. abba n. sp. ex H. platessoides from D. varicus s. s. (= DV1 [26]) from these hosts from the White Sea, Barents Sea (Russia) and Scandinavian waters of the North Sea (Sweden and Norway) was 3%. There was 2% divergence between the sequences of D. abba n. sp. and those of D. varicus DV3 [30] recovered from E. fedorovi in the North Pacific [63]. They differed from those of D. ruber ex Chelidonichthys lastoviza off the coast of Algeria by 3%. The largest divergence was shown between the sequences of D. abba n. sp. and those of D. lacustris from Argentinean freshwater fishes [67], reaching up to 10%. The divergence between D. abba n. sp. and P. muelleri from different White Sea fishes [30] was 8%.
In the phylogenetic tree of 28S rDNA sequences (Fig. 6), D. abba n. sp. from Svalbard (ex H. platessoides) clustered as a well-supported clade with D. varicus DV2 ex the same host from the North Sea [46] and ex B. scalariforme from the White Sea [30]. This clade was well separated from the D. varicus s. s. clade ex multiple hosts from the White Sea, Barents Sea (Russia) and Scandinavian waters of the North Sea (Sweden and Norway), the DV3 clade (ex E. fedorovi), the D. lacustris clade (ex G. maculatus), and the D. ruber clade (ex C. lastoviza).
The ITS2 tree was constructed using 36 sequences of the Derogenidae (Fig. 7). The trimmed matrix included 428 positions. The newly generated sequences of D. abba n. sp. from Svalbard (ex H. platessoides) were identical to those of D. varicus DV2 ex intermediate hosts from the White Sea and Barents Sea (Russia). They differed by 4% from the sequences of D. varicus s. s. from the White Sea, Barents Sea (Russia), and Scandinavian waters of the North Sea (Sweden and Norway) and those of D. ruber off Algeria (ex C. lastoviza). The sequences of D. abba n. sp. from Svalbard (ex H. platessoides) formed a well-supported clade with those of D. varicus DV2. This clade separated from the D. varicus s. s. clade and the D. ruber clade.
 |
Figure 7 Tree inferred using the maximum likelihood method based on the ITS2 sequence data; only bootstrap values higher than 70 are indicated. |
The cox1 sequences of D. abba n. sp. from Svalbard (ex H. platessoides) were aligned with 31 other derogenid sequences, all relating to the genera Derogenes and Progonus. The trimmed matrix included 783 positions. Two of them were identical, while these sequences differed by 1% from those of D. varicus DV2 from the White and Barents seas off Russia (ex intermediate hosts). The divergence was 21–31% between the sequences of D. abba n. sp. and those of D. varicus s. s. [3, 30] and was 21–24% between the former and those of D. ruber from Algerian C. lastoviza [18]. The highest divergence to a congener was 38–39% from D. lacustris from Argentinean freshwater fishes [67]. In the phylogenetic tree of cox1 (Fig. 8), the sequences of D. abba n. sp. clustered with those of D. varicus DV2 within a well-supported clade. This clade was well separated from the D. ruber clade and the D. lacustris clade.
 |
Figure 8 Tree inferred using the maximum likelihood method based on the cox1 sequences; only bootstrap values higher than 70 are indicated. |
Discussion
Differential diagnosis for Derogenes abba n. sp.
WoRMS [71] lists 26 species of the genus Derogenes from the Mediterranean, Atlantic, Pacific, Indian Ocean and the Antarctic. Derogenes bohaiensis Qiu & Liang, 1995, D. crassus Manter, 1934, D. infirmus (Linton, 1940), D. lacustris Tsuchida, Flores, Viozzi, Rauque & Urabe, 2021, and D. varicus (Müller, 1784) were all first described from Atlantic waters [36, 42, 46, 59, 61, 67]. Derogenes adriaticus Nikolaeva, 1966, D. affinis (Rudolphi, 1819), D. bonnieri (Monticelli, 1893), D. latus Janiszewska, 1953, D. minor Looss, 1901, D. ruber Lühe, 1900, and D. robustus Brinkmann, 1966 were all first described from the Mediterranean [9, 25, 39, 40, 44, 48, 55]. Derogenes capricorniensis Bray, 1989, D. chelidonichthydis Shen, 1989, D. epinepheli Wang, 1982, D. magnus Wang, 1991, D. gadi Shen, 1990, D. macrostoma Yamaguti, 1938, D. nototheniae Manter, 1954, D. pharyngicola Bray, Cribb & Barker, 1993, D. minoi Shen, 1990, and D. pearsoni Bray, Cribb & Barker, 1993 were all first described from the Pacific [5, 7, 43, 58, 60, 69, 70, 72]. Derogenes hyderabadensis Jaiswal, 1967 and D. indicus (Singh, 1979) were described from the Indian Ocean [24, 62]. The only known species from the Antarctic is D. johnstoni Prudhoe & Bray, 1973 [54]. Their hosts, collection localities, number of specimens measured and morphometrical data are summarised in Tables 3–5. According to Bray et al. [7], the species of Derogenes can be allocated to two groups, based on egg size. We updated the lists given by Tsuchida et al. [67], and the 26 Derogenes species accepted on WoRMS [71] can be divided as follows: the small egg group with eggs <40 μm comprises eight species: D. adriaticus, D. capricorniensis, D. indicus, D. epinepheli, D. macrouri, D. nototheniae, D. pearsoni, and D. pharyngicola. The large egg group comprises 17 species with eggs >40 μm which are: D. affine, D. bohaiensis, D. chelidonichthydis, D. crassus, D. fuhrmanni, D. gadi, D. johnstoni, D. latus, D. infirmus, D. macrostoma, D. magnus, D. minoi, D. minor, D. ruber, D. robustus, D. parvus, and D. varicus. For D. bonnieri only body length was given [44], and thus we cannot assign it to any group. As eggs of D. abba n. sp. are over 40 μm, it is here considered relative to the second group. Krupenko et al. [30] provided morphometric data from molecularly identified adult D. varicus s. s. and a single D. varicus DV2. Derogenes abba n. sp. ex H. platessoides from Svalbard were similar to the specimen studied by Krupenko et al. [30], but egg dimensions differed. The eggs of the single D. varicus DV2 specimen from the stomach of the whelk B. scalariforme measured 57–63 × 32–38 μm, while those from the present specimens were smaller (40–52 × 28–34 μm). However, the trematodes from the White- and Barents Sea gastropods and those from Svalbard H. platessoides showed no difference in 28S or ITS2 sequences and differed only by a single substitution in the cox1 gene. Derogenes abba n. sp. ex H. platessoides differed from D. varicus s. s. by 3% in 28S, by 4% in ITS2, and by 21–31% in cox1. This is strong evidence that D. abba n. sp. ex H. platessoides and D. varicus s. s. are distinct species.
In the following section, we compare the new species D. abba n. sp. with five species of the large egg group first described from the Atlantic (Table 3), five species first described from the Mediterranean (Table 4), and seven species first described from the Pacific, Antarctic, and Indian Oceans (Table 5).
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Mediterranean. *Diameter. 1We included measurements from the redescription of Rudolphi’s specimen by Lühe (1901) as the original description did not provide any measurements. 2Ratio given by Brinkmann (1967). For the 6th Mediterranean species D. bonnieri (Monticelli, 1893) from Eutrigla gurnardus off Wimereux, France [44], only body length (1500–2000) was given.
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Pacific, Antarctic, and Indian Ocean. *Diameter.
Comparison with Atlantic species (Table 3)
Derogenes abba n. sp. can be distinguished from D. bohaiensis by having a larger body (1100–1450 × 370–450 vs. 782–1003 ×255–323), and a larger sinus sac (93–102 × 84–99 vs. 51–68 × 17–34). It can also be distinguished from D. bohaiensis by having Drüsenmagen which are lacking in D. bohaiensis. Derogenes abba n. sp. differs from D. crassus by having a smaller body (1100–1450 × 370–450 vs. 2268 × 882), smaller ventral (350 vs. 697) and oral (150 vs. 285) suckers, and by the extension of uterine coils which fills most of the body in D. crassus vs. not extending to body edges in D. abba n. sp. Derogenes crassus can be easily differentiated from D. abba n. sp. by its vitellaria extended transversally rather than longitudinally. Derogenes abba n. sp. differs from D. infirmus by forebody length (396–485 vs. 940–1170), and in having slightly smaller oral (95–180 × 110–225 vs. 290–240 × 290–310) and ventral (226–425 × 210–430 vs. 420–490× 359–490) suckers. They can be easily distinguished by the lack of post-gonadal uterine distribution in D. infirmus.
Derogenes abba n. sp. can be distinguished from D. lacustris by having a longer pars prostatica, larger oral sucker, larger pharynx, larger vitelline masses, and visibly larger eggs (see Tables 4 and 5 in Tsuchida et al. [67]). In addition, the divergence between D. abba n. sp. and D. lacustris ranged between 10% in 28S and reached 38–39% in cox1, in addition to reciprocal monophyly in both trees (Figs. 6 and 8).
As discussed below, we could find no morphological basis for the separation of D. abba n. sp. from D. varicus s.s.
Comparison with Mediterranean species (Table 4)
Derogenes abba n. sp. differs from D. affinis by having larger oral (95–180 × 110–225 vs. 190) and ventral (226–425 × 210–430 vs. 250) suckers and larger eggs (40–52 × 28–34 vs. 45 × 20). Derogenes abba n. sp. differs from D. latus by having markedly smaller body organs (see Table 4). These species also differ in the vitellaria shape, which are deeply lobed in D. latus vs. entire in D. abba n. sp.; and by the posterior end, being rounded in our specimens vs. sharply narrowing in D. latus. Derogenes abba n. sp. most closely resembles D. minor in the position of the seminal vesicle, length of pars prostatica, and the position and dimensions of the suckers but the two are distinguished by the posterior end being pointed in D. minor vs. rounded in D. abba n. sp and by the shape of vitelline masses, being lobed and “mulberry shaped” in D. minor vs. entire in D. abba n. sp. Derogenes abba n. sp. shares a sausage-shaped body with D. robustus. Derogenes abba n. sp differs from D. robustus by being smaller (1100–1450 × 370–450 vs. 2600 × 850) with a smaller ventral sucker (226–425 × 210–430 vs. 550), the anterior extent of uterine coils (present in mid-forebody in D. abba n. sp. vs. absent in mid-forebody in D. robustus) and the shape of seminal vesicle (saccular in D. abba n. sp. vs. sausage-shaped in D. robustus).
Derogenes abba n. sp. can be distinguished from D. ruber by the position of the testes (preovarian in D. ruber vs. postovarian in D. abba n. sp.) and by the lobed vitellarium of D. ruber being lacking in D. abba n. sp. Derogenes abba n. sp. and D. ruber can also be distinguished by the body length (1100–1450 in D. abba n. sp. vs. 5000–6000 in D. ruber), by having much smaller suckers (oral, 95–180 in D. abba n. sp. vs. 600 in D. ruber; ventral, 226–425 in D. abba n. sp. vs. 750 in D. ruber) and smaller vitelline masses in D. abba n. sp. The genetic divergence between D. abba n. sp. and D. ruber ranged from 3% in 28S and to 21–24% in cox1. The two species also clustered in distinct clades (Figs. 6–8).
Comparison with species from the Pacific (Table 5)
Derogenes abba n. sp. differs from D. minoi in smaller body size and organs (see Table 5). The two species differ by the indented vitelline masses in D. minoi vs. unlobed masses in D. abba n. sp. Derogenes magnus resembles D. abba n. sp. in also having a sausage-shaped body. Derogenes abba n. sp. can be distinguished from D. magnus by being smaller (1100–1450 × 370–450 vs. 4000 × 1200), with smaller suckers, pharynx, seminal vesicle testes, ovary, and vitelline masses.
Derogenes abba n. sp. differs from D. chelidonichthydis by having a smaller body (1150 vs. 2091), smaller pharynx (75 × 75 vs. 50 × 102), larger sinus-sac (95 × 80 vs. 85 × 65), smaller seminal vesicle (75 × 54 vs. 119 × 85), and larger testes. The two species can also be distinguished by the posterior end being sharply pointed in D. chelidonichthydis vs rounded in D. abba n. sp.
From D. gadi, D. abba n. sp. can be differentiated by having a shorter body (1100–1450 vs. 3332), a shorter forebody, a shorter pars prostatica, a smaller ventral sucker (226–425 vs. 561), a smaller ovary (120–182 × 110–168 vs. 170 × 204) and smaller vitelline masses.
Derogenes abba n. sp. differs from D. macrostoma by having a smaller body (1100–1450 × 370–450 vs. 3000–3200 × 700–950), smaller oral (95–180 × 110–225 vs. 440–460 × 510) and ventral (226–425× 210–430 vs. 750–800) suckers, and a smaller ovary (120–182 × 110–168 vs. 188–225 × 250–260).
Comparison with species from the Antarctic (Table 5)
Derogenes abba n. sp. differs from D. johnstoni by having a smaller ventral sucker (226–425× 210–430 vs. 500–530), smaller eggs (length 40–63 vs. 62–72) and by the shape and size of the seminal vesicle (oval, 56–80 × 38–65 vs. elongate, 360 × 120).
Comparison with species from the Indian Ocean (Table 5)
Derogenes abba n. sp. differs from D. hyderabadensis by having a smaller body (1147–1882 × 216–499 vs. 2560 × 650), smaller oral (130–223 × 132–235 vs. 320 × 280) and ventral (251–384 × 251–359 vs. 570 × 530) suckers, smaller vitelline masses (see Table 5) and larger eggs (48–58 × 30–52 vs. 31–41 × 14–16). It can also be distinguished by having the uterine coils reaching the post-vitelline field, whereas post-vitelline uterine coils are lacking in D. hyderabadensis.
Derogenes abba n. sp. as a cryptic species
Genetically, Krupenko et al. [30] split D. varicus species complex into 4 groups: DV1–DV4. Bouguerche et al. [3] strongly showed that the DV1 clade is the “real” D. varicus and proposed to recognise it as D. varicus s. s. through the analysis of the 28S sequences of D. varicus from the type-host, the Atlantic salmon S. salar. Krupenko et al. [30] did not find any adult stages of DV2 in fish and hence could not provide further morphological comparison of DV1 and DV2. Additionally, the only available sequences of D. varicus s. s. from the type-host had been made available only recently [3]. Herein, the sequences of D. abba n. sp. from Arctic H. platessoides were identical to those of D. varicus s. l. from the same host in the North Sea [50] and those of D. varicus DV2 as rediae from gastropods in the White and Barents seas [30]. They were also identical to D. varicus of Olson et al. [50] (AY222189) ex H. platessoides from the North Sea. On the other hand, they differed from the sequence of D. varicus s. s. from Norwegian S. salar by 3% as p-distance. This level of divergence is comparable to that observed between the well-established Mediterranean species D. ruber ex C. lastoviza from the Western Mediterranean, off Algeria (3% as well). Thus, the genetic divergence supports that D. abba n. sp. is a separate species from D. varicus s. s. This species uses a different first intermediate host, and produces cercariae that can be morphologically distinguished from those of D. varicus s. s. [30].
Krupenko et al. [30] speculated that D. varicus DV2 is the species that Shulman and Shulman-Albova (1953) called D. crassus Manter, 1934. Type localities for D. crassus sensu Manter (1934) and of D. abba n. sp. are so distinct (Tortugas, Florida, western Atlantic for D. crassus [42] vs. Svalbard, Arctic Norway, for D. abba n. sp.) that we consider this unlikely. However, it remains possible that D. crassus sensu Shulman and Shulman-Albova (1953) represents D. abba n. sp.
The lineage D. varicus DV4 corresponds to an 18S rDNA sequence (AJ287511) of D. varicus of Littlewood and Olson [37] from the same host as D. abba n. sp., H. platessoides from the North Sea. DV4 was labeled only based on the genetic divergence of 0.13% (p-distance) from D. abba n. sp. (DV2) in the 18S rDNA sequence [30]. In light of the available data, it is premature to consider D. varicus DV4 as a distinct species from D. abba n. p. considering that our study does not include the 18S rDNA analysis. Hence, we cannot estimate the taxonomical position of DV4 further. Meanwhile, the possibility that D. varicus (s.s.) also occurs in H. platessoides is not ruled out.
Overall, the evolution of understanding of this genus is such that while most combinations of species can be distinguished on the basis of morphology, some are presently morphologically cryptic with respect to each other. On this basis, it remains critical that further study is based on both morphological and molecular data. A key area of uncertainty relates to patterns of host-specificity. Some species, e.g. D. varicus s.s., appear to be genuinely euryxenic, whereas several others apparently have highly restricted host ranges. The extent to which this is a genuine reflection of the true nature of these species is uncertain. Certainly, for clearly closely related species, it is not obvious why host-specificity patterns should differ so dramatically.
The “lost collections of A. Looss”: Mediterranean Derogenes spp.
The German parasitologist Prof. Arthur Looss (1861–1923) was among one of the most prolific parasitologists and taxonomists and was known for his “enthusiasm and energy as a researcher that have probably seldom been surpassed, especially a painstaking attention to detail that is unfortunately rare” [33]. After his death, his collection was divided between numerous institutions: Smithsonian National Museum of Natural History in Washington (USA), the Natural History Museum in Berlin and the Natural History Museum in Leipzig (Germany), Gothenburg Museum of Natural History and the Swedish Museum of Natural History (Sweden) (see Kuzmina and Holovachov [31]). A part of Looss’s collection including his archives was sold to the Swedish Museum of Natural History (Naturhistoriska riksmuseet) in Stockholm by his widow, Elise Looss in 1924 [31] and includes slides of which some are actually type material and vials containing trematodes preserved in ethanol, along with several publication-ready drawings and original line drawings.
One of the intriguing derogenids that we encountered in this collection, is “D. limula” which we described above as Derogenes sp., ex P. tentacularis, collected from off Trieste, Italy, Central Mediterranean. Curiously, we also found line drawings by A. Looss (see Figs. 3A and 3B) labeled as “Derogenes limula”, which suggests that he intended to describe Derogenes from P. tentacularis as a new species, with the name “D. limula”. We found one specimen (SMNH 208361) for which the measurements are presented in Table 6. The eggs of this “D. limula” that we described above as Derogenes sp. are over 40 μm and the species is thus consistent with the “large eggs group”. We compared the measurements of the sole specimen of Derogenes ex P. tentacularis to those of congeneric species from the Mediterranean (Table 6). The single specimen of Derogenes sp. (or “D. limula” as initially referred to by A. Looss on the illustrations) ex P. tentacularis differs from D. minor, D. robustus, D. affine, and D. latus by its larger eggs. It resembles D. ruber in egg size (61 × 39 in Derogenes sp. vs. 62 × 39 in D. ruber) and in having lobed, tear-shaped vitelline masses. However, Derogenes sp. ex P. tentacularis differ from D. ruber by being smaller in all body measurements including the body (855 × 253 vs. 7869 × 1847). We note though that body size is not a sufficient differentiating character and can vary with the age of the worm and the suitability of the host.
Measurements of Derogenes sp. “limula”. from Parablennius tentacularis off Trieste, Italy and Derogenes spp. first described from the Mediterranean. *Diameter. 1We included measurements from the redescription of Rudolphi’s specimen by Lühe (1901) as the original description did not provide any measurements.
The presence of Derogenes sp. in P. tentacularis is not unusual as there are previous records of Derogenidae in blenniid fishes. For instance, Mediterranean blenniid host species of the related halipegine derogenid genus Magnibursatus Naidenova, 1969 [29, 51]. The peacock blenny Salaria pavo is reported as host for M. skrjabini (Vlasenko, 1931) [2, 29] and for M. blennii (Paggi & Orecchia, 1975) [29], whereas the tompot blenny P. gattorugine, the rusty blenny P. sanguinolentus, the ‘futarra’ Paralipophrys trigloides and Parablennius sp. are also hosts for M. blennii [29, 51]. Although we have only two specimens, the presence of an external seminal vesicle (vs. internal in Magnibursatus [20]), a uterus extending posterior to vitelline masses (vs. almost entirely anterior to vitellarium in Magnibursatus [20]), having lobed vitelline masses (vs. entire in Magnibursatus [20]) and especially the eggs lacking filaments (vs. present at each end in Magnibursatus [20]) indicate that the derogenid collected by A. Looss agrees with Derogenes rather than Magnibursatus.
Lebour [34] described some D. varicus adults with a similar “spiny” appearance and stated “… curious fact noticed is that all these larval D. varicus are beset with small spines, whereas it is a characteristic of the adult that although it has sometimes a wrinkling of the skin, it is unarmed and usually smooth. It is possible that these wrinkles may be the remains of the spines fused together. The spines are especially distinct in the younger specimens.” Interestingly, some of her specimens also contained eggs (i.e., progenetic). She also showed this spination in her figures [34]. Hence, this “D. limula” that we described as Derogenes sp., is arguably an immature D. ruber. We could not find any published records for Derogenes in Blenniidae in the Mediterranean, but D. varicus was recorded from the butterfly blenny Blennius ocellaris in the Atlantic [47]. Besides the localities being distant (Trieste, Mediterranean for “D. limula” from P. tentacularis vs. Plymouth, Atlantic for D. varicus of Nicoll [47]), “D. limula” from P. tentacularis can be readily distinguished from D. varicus by having lobed vitelline masses. Hence, “D. limula” of A. Looss is clearly not D. varicus. Herein, we described it as Derogenes sp. pending further examination based on more specimens.
Progonus muelleri (Levinsen, 1881) and Derogenes varicus sensu lato
The monotypic genus Progonus was erected by Looss [38] for P. muelleri, first described from waters off Greenland [35]. The two genera Derogenes and Progonus are very similar with the only difference being the presence of a cyclocoel in P. muelleri vs. blindly ending caeca in species of Derogenes [19, 35, 49]. We examined body sections of representatives of both genera, mainly D. varicus s. s. from Limanda limanda (Fig. 5C) and P. muelleri from M. scorpius and the two species can also be readily distinguished by the seminal vesicle, located in the anterior third of the midbody of D. varicus s. s. vs. in the posterior third of the midbody in P. muelleri. Additionally, the pars prostatica is far shorter in P. muelleri (see Fig. 5).
We examined additional specimens of Derogenes spp. from T. Odhner’s collections at the Invertebrates collection in the SMNH (Figs. 9 and 10), all identified and labelled as D. varicus, which we consider herein as representative of the D. varicus species complex or D. varicus s. l. The hosts were Argentina sphyraena, Brosme brosme, Molva molva, and Platichthys flesus. Corresponding morphometrical data are indicated in Table 7. Overall, specimens from the previously mentioned hosts share the same anatomical features, concerning the organisation of gonads and genital terminalia (Figs. 9B, 9D, 9F, 9H, 10B). Derogenes varicus s. l. from the previously mentioned hosts share the sausage-shaped appearance, except D. varicus s. l. from B. brosme that has a stockier appearance. The testes in D. varicus s. l. from A. sphyraena and the one from H. hippoglossus differ by having more longitudinally elongated testes. The most striking one is D. varicus s. l. from A. sphyraena that also differed slightly by the organisation of the genital terminalia (Fig. 9B), the shape of vitelline masses being larger and elongated, by having a smaller body and smaller organs but the measurements of eggs overlapped. However, the number of specimens measured from T. Odhner’s collection is low and thus we refrained from comparing morphometrical values. Since we are attempting to delineate species within the D. varicus species complex using integrative taxonomy, and as molecular sequence data are lacking for Derogenes from A. sphyraena, B. brosme, M. molva, and P. flesus, we consider specimens from the previously mentioned hosts as D. aff. varicus or D. varicus s. l., pending further examinations.
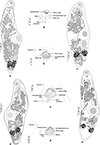 |
Figure 9 Derogenes varicus sensu lato. A, whole body ex Argentina sphyraena (SMNH-114558). B, terminal genitalia ex A. sphyraena (SMNH-114558). C, whole body ex Brosme brosme (SMNH-114560). D, terminal genitalia ex B. brosme (SMNH-114560). E, whole body ex Platichthys flesus (SMNH-208360). F, terminal genitalia ex P. flesus (SMNH-208360). G, whole body ex Molva molva (SMNH-114559). H, terminal genitalia ex M. molva (SMNH-114559). |
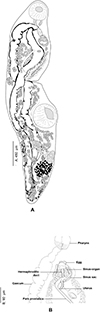 |
Figure 10 Derogenes varicus sensu lato ex Hippoglossus hippoglossus (SMNH-104577). A, whole body. B, terminal genitalia. |
Measurements of D. varicus sensu lato from T. Odhner’s collection examined in the present study.
Acknowledgments
We thank the participants of the HHUMTL22 cruise, the crew of the R/V “Helmer Hanssen”, the participants of the International Bottom Trawl Survey cruises, and the crew of the R/V “Svea” for their assistance with sampling. Special thanks to Barbara Bland “Institutionen för akvatiska resurser” (SLU Aqua), Andreas Altenburger (UiT) and Bo Delling (SMNH) for kindly helping with fish acquisition. We thank the staff of the Kristineberg Center for Marine Research and Innovation for their assistance. We thank the reviewers for their constructive and detailed comments, especially Thomas H. Cribb (the University of Queensland, School of Biological Sciences, Australia) for his close reading of our article. Special thanks to Gerardo Perez-Ponce de Leon (Escuela Nacional de Estudios Superiores unidad Mérida, Mexico) for discussing the earlier drafts of the molecular analysis and his support and guidance; and Prof. Jean-Lou Justine (Muséum National d’Histoire Naturelle, France) for assisting with the etymology. Special thanks to Philipp Schiffer (University of Cologne, Germany), Lyliane Schiffer and Marcus, Agnetha, and Mickael Forsberg for their help with logistics. Thanks are extended to Martin Vinther Sørensen (SNM) for the loan of specimens. We thank Laura Pavesi (SNM) and Vanessa Pitusi (SNM) for providing accession numbers.
Funding
This research was funded by the Swedish Taxonomy Initiative, Artdatabanken, Swedish University of Agricultural Sciences within the scope of the project “Taxonomy and systematics of digenetic trematodes parasitising fishes of Sweden” (dha 2019.4.3-48). Specimens from the Arctic Ocean were collected during the HHUMTL22 cruise organised and partly funded by the Arctic University Museum of Norway.
Conflicts of Interest
Authors have no potential conflict of interest.
References
- Ahuir-Baraja AE, Fraija-Fernández N, Raga JA, Montero FE. 2015. Molecular and morphological differentiation of two similar species of Accacoeliidae (Digenea): Accacladocoelium macrocotyle and A. nigroflavum from sunfish, Mola mola. Journal of Parasitology, 10, 231–235. [CrossRef] [PubMed] [Google Scholar]
- Bartoli P, Gibson DI, Bray RA. 2005. Digenean species diversity in teleost fish from a nature reserve off Corsica, France (Western Mediterranean), and a comparison with other Mediterranean regions. Journal of Natural History, 39, 47–70. [Google Scholar]
- Bouguerche C, Huston DC, Cribb TH, Karlsbakk E, Ahmed M, Holovachov O. 2023. Hidden in the fog: morphological and molecular characterisation of Derogenes varicus sensu stricto (Trematoda, Derogenidae) from Sweden and Norway, and redescription of two poorly known Derogenes species. Parasite, 30, 35. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Bray RA. 1979. Digenea in marine fishes from the eastern seaboard of Canada. Journal of Natural History, 13, 399–431. [CrossRef] [Google Scholar]
- Bray RA. 1989. Derogenes capricorniensis, sp. nov. (Digenea, Derogenidae), in a Blenny From Heron Island. Queensland. Australian Journal of Zoology, 37, 55–58. [CrossRef] [Google Scholar]
- Bray RA, Cribb TH. 2015. Are cryptic species a problem for parasitological biological tagging for stock identification of aquatic organisms? Parasitology, 125–133. [CrossRef] [PubMed] [Google Scholar]
- Bray RA, Cribb TH, Barker SC. 1993. The Hemiuroidea (Digenea) of pomacentrid fishes (Perciformes) from Heron Island, Queensland, Australia. Systematic Parasitology, 24, 159–184. [CrossRef] [Google Scholar]
- Bray RA, Cutmore SC, Cribb TH. 2022. A paradigm for the recognition of cryptic trematode species in tropical Indo-west Pacific fishes: the problematic genus Preptetos (Trematoda: Lepocreadiidae). International Journal for Parasitology, 52, 169–203. [CrossRef] [PubMed] [Google Scholar]
- Brinkmann A. 1967. Some trematodes from marine fishes in the waters of Rhodes. Acta Universitatis Bergensis, Series Mathematica Rerumque Naturalium, 10, 3–13. [Google Scholar]
- Calhoun DM, Curran SS, Pulis EE, Provaznik JM, Franks JS. 2013. Hirudinella ventricosa (Pallas, 1774) Baird, 1853 represents a species complex based on ribosomal DNA. Systematic Parasitology, 86, 197–208. [CrossRef] [PubMed] [Google Scholar]
- Cháves-González LE, Morales-Calvo F, Mora J, Solano-Barquero A, Verocai GG, Rojas A. 2022. What lies behind the curtain: Cryptic diversity in helminth parasites of human and veterinary importance. Current Research in Parasitology & Vector-borne Diseases, 2, 100094. [CrossRef] [PubMed] [Google Scholar]
- Cribb TH. 2016. Editorial: The biodiversity of trematodes of fishes. Systematic Parasitology, 93, 219–221. [CrossRef] [PubMed] [Google Scholar]
- Cribb TH, Adlard RD, Bray RA. 1998. A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). International Journal for Parasitology, 28, 1791–1795. [CrossRef] [PubMed] [Google Scholar]
- Cribb TH, Bray RA. 2010. Gut wash, body soak, blender, and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7. [CrossRef] [PubMed] [Google Scholar]
- de León GP-P, Hernández-Mena D. 2019. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the “next-generation” Tree of Life. Journal of Helminthology, 93, 260–276. [CrossRef] [PubMed] [Google Scholar]
- de León GP-P, Nadler SA. 2010. What we don’t recognize can hurt us: a plea for awareness about cryptic species. Journal of parasitology, 96, 453–464. [CrossRef] [PubMed] [Google Scholar]
- García-Varela M, Nadler SA. 2005. Phylogenetic relationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rDNA gene sequences. Journal of Parasitology, 91, 1401–1409. [CrossRef] [PubMed] [Google Scholar]
- Gharbi K, Bouguerche C, Ahmed M, Pérez-Ponce de León G, Tazerouti F. 2023. Redescription and Molecular Characterisation of Derogenes ruber Lühe, 1900 (Hemiuroidea: Derogenidae) from Chelidonichthys lastoviza (Scorpaeniformes: Triglidae) in the Western Mediterranean. Acta Parasitologica, 69, 309–323. [Google Scholar]
- Gibson DI. 1996. Guide to the parasites of Canadian fishes. Part IV. Trematoda. Canadian Special Publication of Fisheries and Aquatic Sciences No. 124. NRC Research Press: Ottawa, Canada. 373 p. [Google Scholar]
- Gibson DI. 2002. Family Derogenidae Nicoll, 1910, in Keys to the Trematoda. Gibson DI, Jones A, Bray RA, Editors. CAB International and the Natural History Museum: Wallingford. p. 351–368. [CrossRef] [Google Scholar]
- Gilardoni C, Etchegoin J, Cribb T, Pina S, Rodrigues P, Diez ME, Cremonte F. 2020. Cryptic speciation of the zoogonid digenean Diphterostomum flavum n. sp. demonstrated by morphological and molecular data. Parasite, 27, 44. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Gordeev II, Sokolov SG. 2020. Helminths of Fedorov’s lumpsucker Eumicrotremus fedorovi Mandrytsa, 1991 (Actinopterygii: Cyclopteridae) in the Simushir Island area (Pacific Ocean). Parasitology International, 76, 102075. [CrossRef] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T-a. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22, 160–174. [CrossRef] [PubMed] [Google Scholar]
- Jaiswal G. 1967. Investigations on the trematode fauna of the common food fishes of Hyderabad, AP Part I. A new species of Derogenes Lühe, 1900, from a freshwater fish, Channa (Ophiocephalus) punctatus. Indian Journal of Helminthology, 8, 36–44. [Google Scholar]
- Janiszewska J. 1953. Some Adriatic Sea fish trematodes. Zoologica Poloniae, 6, 20–48. [Google Scholar]
- Justine J-L, Briand MJ, Bray RA. 2012. A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitology Research, 111, 341–351. [CrossRef] [PubMed] [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. [CrossRef] [PubMed] [Google Scholar]
- Køie M. 2000. Metazoan parasites of teleost fishes from atlantic waters off the Faroe Islands. Ophelia, 52, 25–44. [CrossRef] [Google Scholar]
- Kostadinova A, Bartoli P, Gibson DI, Raga JA. 2004. Redescriptions of Magnibursatus blennii (Paggi & Orechhia, 1975) n. comb. and Arnola microcirrus (Vlasenko, 1931) (Digenea: Derogenidae) from marine teleosts off Corsica. Systematic Parasitology, 58, 125–137. [CrossRef] [PubMed] [Google Scholar]
- Krupenko D, Kremnev G, Gonchar A, Uryadova A, Miroliubov A, Krapivin V, Skobkina O, Gubler A, Knyazeva O. 2022. Species complexes and life cycles of digenetic trematodes from the family Derogenidae. Parasitology, 149, 1590–1606. [CrossRef] [PubMed] [Google Scholar]
- Kuzmina T, Holovachov O. 2023. Equine Strongylidae and other parasitic nematodes described by Arthur Looss during 1895–1911 in the collections of the Swedish Museum of Natural History. Zootaxa, 5227, 151–193. [CrossRef] [PubMed] [Google Scholar]
- Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics, 30, 3276–3278. [CrossRef] [PubMed] [Google Scholar]
- Lawrence RA. 1989. Larva Migrans then. American Journal of Tropical Medicine and Hygiene, 41, 18–20. [Google Scholar]
- Lebour MV. 1917. Some parasites of Sagitta bipunctata. Journal of the Marine Biological Association of the United kingdom, 11, 201–206. [CrossRef] [Google Scholar]
- Levinsen GMR. 1881. Bidrag til kundskap om Gronlands trematodfauna. Oversigt over det kongelige Dansk videnskadernes Selskabs Forhandlinger, 23, 52–84 (in Danish). [Google Scholar]
- Linton E. 1940. Trematodes from fishes mainly from the Woods Hole region, Massachusetts. Proceedings of the United States National Museum, 88, 69–102. [CrossRef] [Google Scholar]
- Littlewood DTJ, Olson PD. 2001. Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise, in Interrelationships of the Platyhelminthes. Littlewood DTJ, Bray RA, Editors. Taylor & Francis: London & New York. p. 262–278. [Google Scholar]
- Looss A. 1899. Weitere Beiträge zur Kenntniss der Trematoden-Fauna Aegyptens, zugleich Versuch einer natürliden Gliederung des Genus Distomum Retzius. Zoologische Jahrbücher Abteilung für Systematik, Geographie und Biologie der Tiere, 12, 521–784 (in German). [Google Scholar]
- Looss A. 1901. Über einige Distomen der Labriden des Triester Hafens. Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten, 29(398–405), 437–442 (in German). [Google Scholar]
- Lühe M. 1900. Über Distomen aus der Gallenblase von Mittelmeerfischen. Zoologischer Anzeiger, 23, 504–509 (in German). [Google Scholar]
- Lühe M. 1901. Ueber Hemiuriden. (Ein Beitrag zur Systematik der digenetischen Trematoden). Zoologischer Anzeiger, 24, 394–403, 473–488 (in German). [Google Scholar]
- Manter HW. 1934. Some digenetic trematodes from deep-water fish of Tortugas, Florida. Papers from Tortugas Laboratory, 28, 257–345. [Google Scholar]
- Manter HW. 1954. Some digenetic trematodes from fishes of New Zealand. Transactions of the Royal Society of New Zealand, 82, 475–568. [Google Scholar]
- Monticelli FS. 1893. Studii sui Trematodi endoparassiti. Primo contributi di osservazioni sui Distomidi. Zoologische Jahrbücher, 3, 1–229 (in Italian). [Google Scholar]
- Morgan J, Blair D. 1995. Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology, 111, 609–615. [CrossRef] [PubMed] [Google Scholar]
- Müller OF. 1784. Zoologia Danica seu Animalium Daniae et Norvegiae rariorum ac minus notorum historia descriptiones et historia vols. 1, 2. Copenhagen & Leipzig: Weygandinis (in Latin). [Google Scholar]
- Nicoll W. 1915. A list of the trematode parasites of British marine fishes. Parasitology, 7, 339–378. [CrossRef] [Google Scholar]
- Nikolaeva VM. 1966. Trematodes of the suborder Hemiurata infecting fish in the Mediterranean Basin, in Helminth fauna of animals of the Southern Seas. Delyamure SL, Editor. Biologiya Morya, Kiev: Russia. p. 52–66 (in Russian). [Google Scholar]
- Odhner T. 1905. Die Trematoden des arktischen Gebietes. Fauna Arctica, 4, 289–372 (in German). [Google Scholar]
- Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ. 2003. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755. [CrossRef] [PubMed] [Google Scholar]
- Paggi L, Orecchia P. 1975. Tyrrhenia blennii new-genus new-species (Hemiurata Halipegidae) parasite of Blennius gattorugine and Blennius sanguinolentus. Parassitologia, 17, 57–64. [PubMed] [Google Scholar]
- Pérez-Ponce de León G, Nadler SA. 2016. The importance of recognising parasite cryptic diversity for research programmes on foodborne trematodiases. Transactions of the Royal Society of Tropical Medicine and Hygiene, 110, 4–5. [CrossRef] [PubMed] [Google Scholar]
- Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M. 2008. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371. [CrossRef] [PubMed] [Google Scholar]
- Prudhoe S, Bray RA. 1973. Digenetic trematodes from fishes. Reports A.N.Z. Antarctic Research Expedition, Series B, 8, 195–225. [Google Scholar]
- Rudolphi CA. 1819. Entozoorum synopsis, cui accedunt mantissa duplex et indices locupletissimi. Berlin: Augusti Rücker. [CrossRef] [Google Scholar]
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B. 2012. Fiji: an open-source platform for biological-image analysis. Nature methods, 9, 676–682. [CrossRef] [PubMed] [Google Scholar]
- Shen JW. 1990. Description of four new species (Lepocreadiidae and Hemiuridae) and a list of digenetic trematodes of fishes from Yellow Sea. Marine Science Bulletin, 9, 54–63 (in Chinese). [Google Scholar]
- Shen JW. 1990. Digenetic trematodes of fishes from the Changjiang River estuary. Studia Marina Sinica, 31, 115–120 (in Chinese with English summary). [Google Scholar]
- Shen JW. 1989. Studies on the digenetic trematodes of fishes from Jiaozhou Bay. Studia Marina Sinica, 30, 153–162 (In Chinese). [Google Scholar]
- Shen JW, Qiu ZZ. 1995. Studies on the trematodes of fishes from the Yellow Sea and the Bo Hai Sea. Beijing: Science Press (in Chinese). [Google Scholar]
- Singh JP. 1979. Liopyge indica n. sp. from a marine fish Chorinemus moadetta (Cuv. and Val.) from Quilon. India. Indian Journal of Zootomy, 20, 51–53. [Google Scholar]
- Sokolov SG, Atopkin DM, Gordeev II. 2021. Phylogenetic position of the hemiuroid genus Paraccacladium Bray & Gibson, 1977 (Trematoda: Hemiuroidea) and the status of the subfamily Paraccacladiinae Bray & Gibson, 1977. Marine Biology Research, 17, 31–40. [CrossRef] [Google Scholar]
- Sokolov SG, Atopkin DM, Urabe M, Gordeev II. 2019. Phylogenetic analysis of the superfamily Hemiuroidea (Platyhelminthes, Neodermata: Trematoda) based on partial 28S rDNA sequences. Parasitology, 146, 596–603. [CrossRef] [PubMed] [Google Scholar]
- Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526. [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. [CrossRef] [PubMed] [Google Scholar]
- Tsuchida K, Flores V, Viozzi G, Rauque C, Urabe M. 2021. Hemiuroidean trematodes from freshwater Patagonian fishes: description of a new species, distribution and molecular phylogeny. Parasitology Research, 120, 1219–1232. [CrossRef] [PubMed] [Google Scholar]
- Vikberg Wernström PJO, Hembrom AA, Slettli Hansen C, Holovachov O, Brenneis G, Zieger E, Wanninger A, Altenburger A. 2022. Cruise Report HHUMTL22: The Arctic University Museum of Norway R/V “Helmer Hanssen” Tromsø–Longyearbyen August 22–29, 2022. Septentrio Reports (1). https://doi.org/10.7557/7.6693 (Original work published September 21, 2022). [Google Scholar]
- Wang PQ. 1991. Report on 1 new genus and 6 new species of digenetic trematodes from marine fishes in Pingtan, Fujian Province. Wuyi Science Journal, 8, 131–138 (in Chinese). [Google Scholar]
- Wang PQ. 1982. Hemiuroid trematodes of marine fishes from Fujian Province, China. Journal of Fujian Teachers University (Natural Science), 2, 67–80 (in Chinese). [Google Scholar]
- WoRMS. 2024. Derogenes Lühe, 1900. Available at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=108722 on 2024-03-08 [Google Scholar]
- Yamaguti S. 1938. Studies on the Helminth Fauna of Japan. Part 21. Trematodes of Fishes, IV. Tokyo: Maruzen. [Google Scholar]
Cite this article as: Bouguerche C, Huston DC, Karlsbakk E, Ahmed M & Holovachov O. 2024. Untangling the Derogenes varicus species complex in Scandinavian waters and the Arctic: description of Derogenes abba n. sp. (Trematoda, Derogenidae) from Hippoglossoides platessoides and new host records for D. varicus (Müller, 1784) sensu stricto. Parasite 31, 26.
All Tables
Fishes examined from Scandinavian waters of the North Sea, Northeast Atlantic and the Arctic Ocean during this study.
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Atlantic. *Diameter. 1Note that D. lacustris is a freshwater species.
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Mediterranean. *Diameter. 1We included measurements from the redescription of Rudolphi’s specimen by Lühe (1901) as the original description did not provide any measurements. 2Ratio given by Brinkmann (1967). For the 6th Mediterranean species D. bonnieri (Monticelli, 1893) from Eutrigla gurnardus off Wimereux, France [44], only body length (1500–2000) was given.
Measurements of Derogenes abba n. sp. from Hippoglossoides platessoides off Svalbard and Derogenes spp. first described from the Pacific, Antarctic, and Indian Ocean. *Diameter.
Measurements of Derogenes sp. “limula”. from Parablennius tentacularis off Trieste, Italy and Derogenes spp. first described from the Mediterranean. *Diameter. 1We included measurements from the redescription of Rudolphi’s specimen by Lühe (1901) as the original description did not provide any measurements.
Measurements of D. varicus sensu lato from T. Odhner’s collection examined in the present study.
All Figures
 |
Figure 1 Derogenes abba n. sp. ex Hippoglossoides platessoides. A, whole body (Type-9562). B, terminal genitalia (Type-9562). C, hologenophore, forebody (Type-9563). |
| In the text | |
 |
Figure 2 “Derogenes limula” ex Parablennius tentacularis (SMNH-208361). |
| In the text | |
 |
Figure 3 “Derogenes limula” ex Parablennius tentacularis. Unpublished line drawing by A. Looss. |
| In the text | |
 |
Figure 4 “Derogenes limula” ex Parablennius tentacularis based on A. Looss’s unpublished line drawings. A, Whole body, ventral view. B, Whole body, lateral view. C, Whole body, ventral view. D, Whole body, ventral view. |
| In the text | |
 |
Figure 5 Progonus muelleri (Levinsen, 1881) ex Myoxocephalus scorpius (NHMD-114950) and comparison between the terminal genitalia in Progonus and in Derogenes. A, P. muelleri ex M. scorpius (NHMD-114950), whole body. B, P. muelleri ex M. scorpius, terminal genitalia, horizontal section (SMNH-114586). C, Derogenes varicus sensu stricto ex Limanda limanda, terminal genitalia, horizontal section (SMNH-114557). |
| In the text | |
 |
Figure 6 Tree inferred using the maximum likelihood method based on the 28S rDNA sequence data; only bootstrap values higher than 70 are indicated. The newly generated sequences of Derogenes varicus sensu stricto from Sweden, Northeast Atlantic are indicated by*. Other D. varicus sensu stricto are those of Krupenko et al. [30] from the Barents Sea and White Sea; and those of Bouguerche et al. [3] from Northeast Atlantic, off Sweden and Norway and from Svalbard, Norway, Arctic Ocean. |
| In the text | |
 |
Figure 7 Tree inferred using the maximum likelihood method based on the ITS2 sequence data; only bootstrap values higher than 70 are indicated. |
| In the text | |
 |
Figure 8 Tree inferred using the maximum likelihood method based on the cox1 sequences; only bootstrap values higher than 70 are indicated. |
| In the text | |
 |
Figure 9 Derogenes varicus sensu lato. A, whole body ex Argentina sphyraena (SMNH-114558). B, terminal genitalia ex A. sphyraena (SMNH-114558). C, whole body ex Brosme brosme (SMNH-114560). D, terminal genitalia ex B. brosme (SMNH-114560). E, whole body ex Platichthys flesus (SMNH-208360). F, terminal genitalia ex P. flesus (SMNH-208360). G, whole body ex Molva molva (SMNH-114559). H, terminal genitalia ex M. molva (SMNH-114559). |
| In the text | |
 |
Figure 10 Derogenes varicus sensu lato ex Hippoglossus hippoglossus (SMNH-104577). A, whole body. B, terminal genitalia. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


