| Issue |
Parasite
Volume 31, 2024
|
|
|---|---|---|
| Article Number | 8 | |
| Number of page(s) | 15 | |
| DOI | https://doi.org/10.1051/parasite/2024006 | |
| Published online | 08 February 2024 | |
urn:lsid:zoobank.org:pub:2539077C-5D44-4EC3-AB59-0AEFFCBCA47C
Research Article
Chewing lice of Bearded Reedling (Panurus biarmicus) and diversity of louse-host associations of birds in reed beds in Slovakia
Mallophages de la Panure à moustaches (Panurus biarmicus) et diversité des associations mallophages-hôtes des oiseaux dans les roselières en Slovaquie
1
Department of Biology and Wildlife Diseases, Faculty of Veterinary Hygiene and Ecology, University of Veterinary Sciences Brno, Palackeho tr. 1946/1, 61242, Brno, Czechia
2
Department of Veterinary Sciences, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kamycka 129, 165 00, Prague 6, Czechia
3
Illinois Natural History Survey, Prairie Research Institute, University of Illinois Urbana-Champaign, IL, 61820, USA
4
Department of Parasitology, Faculty of Science, University of South Bohemia, České Budějovice, Czechia
5
Institute of Parasitology, Biology Centre, Czech Academy of Sciences, v. v. i, České Budějovice, Czechia
6
Department of Biology, University of Trnava, Priemyselna 4, 918 43, Trnava, Slovakia
7
Institute of Vertebrate Biology, Czech Academy of Sciences, v. v. i, Kvetna 8, 603 65, Brno, Czechia
* Corresponding author: sychrao@vfu.cz
Received:
10
October
2023
Accepted:
19
January
2024
A total of 1,621 wild birds representing 34 species were examined for chewing lice in reed beds in southwestern Slovakia during the pre-breeding migration 2008–2009 and 2016–2019. A total of 377 (23.3%) birds representing 15 species were parasitized by 26 species of chewing lice of 12 genera. Dominant genera were Penenirmus (with dominance 32.6%) and Menacanthus (29.4%), followed by Brueelia (12.6%), Acronirmus (10.8%), Philopterus (7.7%), and Myrsidea (4.2%). We evaluated 33 host-louse associations including both 1) host-generalist, parasitizing more than one host species and host-specific lice, occurring only on a single host species, and 2) lice species with large range geographic distribution, reported across the range of the distribution of their hosts and lice species with only occasional records from a limited area within the range of their hosts. The Bearded Reedling, Panurus biarmicus (Linnaeus, 1758), was parasitized by two species of chewing lice, Menacanthus brelihi Balát, 1981 and Penenirmus visendus (Złotorzycka, 1964), with conspicuously different prevalences (5.6% vs. 58.2%, respectively; n = 251). New material enabled us to redescribe both species of lice: the first one is resurrected from previous synonymy as a valid species. A fragment of the mitochondrial cytochrome oxidase I gene was sequenced from these two species in order to assess their relative phylogenetic position within their genera. Our study demonstrates the importance of an adequate identification of parasites, especially on rarely examined and endangered hosts.
Résumé
Au total, 1 621 oiseaux sauvages représentant 34 espèces ont été examinés à la recherche de mallophages dans les roselières du sud-ouest de la Slovaquie au cours de la migration de pré-reproduction 2008–2009 et 2016–2019. Parmi ceux-ci, 377 oiseaux (23,3 %), représentant 15 espèces, étaient parasités par 26 espèces de mallophages de 12 genres. Les genres dominants étaient Penenirmus (avec une dominance de 32,6 %) et Menacanthus (29,4 %), suivis de Brueelia (12,6 %), Acronirmus (10,8 %), Philopterus (7,7 %) et Myrsidea (4,2 %). Nous avons évalué 33 associations mallophage-hôte comprenant à la fois 1) des espèces de mallophages généralistes, parasitant plus d’une espèce hôte, et des mallophages spécifiques, présents uniquement sur une seule espèce hôte et 2) des espèces de mallophages ayant une large répartition géographique, signalées à travers l’étendue de la répartition de leurs hôtes, et des espèces de mallophages avec seulement des observations occasionnelles dans une zone limitée à l’intérieur de l’aire de répartition de leurs hôtes. La Panure à moustaches, Panurus biarmicus (Linnaeus, 1758), était parasitée par deux espèces de mallophages, Menacanthus brelihi Balát, 1981 et Penenirmus visendus (Złotorzycka, 1964), avec des prévalences nettement différentes (respectivement 5,6 % et 58,2 %, n = 251). Du nouveau matériel nous a permis de redécrire les deux espèces de mallophages, la première étant ressuscitée de la synonymie précédente en tant qu’espèce valide. Un fragment du gène mitochondrial de la cytochrome oxydase I a été séquencé à partir de ces deux espèces afin d’évaluer leur position phylogénétique relative au sein de leurs genres. Notre étude démontre l’importance d’une identification adéquate des parasites, en particulier sur les hôtes rarement examinés et menacés.
Key words: Menacanthus / Penenirmus / Redescription / Prevalence / Intensity
© O. Sychra et al., published by EDP Sciences, 2024
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A diverse assemblage of chewing lice (Phthiraptera) and their host associations are well-known from Slovakia. Recently we reviewed all available published records, old museum collections, and recently collected material of chewing lice from Slovakia and provided a checklist containing 249 species of chewing lice – 65 amblyceran species from 22 genera representing the families Laemobothriidae, Menoponidae, and Ricinidae, and 184 ischnoceran species from 54 genera of the family Philopteridae – and 358 host-louse associations from 171 bird species from 21 orders [36]. Nevertheless, information about infestation characteristics like prevalence or mean intensity is still scarce, incomplete, and focused on louse species from hosts occurring at higher elevations, in montane forests and shrublands [2, 8, 17–20].
We recently focused on a lowland wetland bird community. We evaluated infestation characteristics of ectoparasites in relation to the migration behavior and sexual dimorphism of five passerine bird species that represent habitat specialists breeding in this environment dominated by Common Reed (Phragmites australis) [51]. In addition to habitat specialists, i.e., Acrocephalus spp., Locustella luscinioides (Savi, 1824), and Panurus biarmicus (Linnaeus, 1758), the avifauna of the reed beds also includes birds that use this environment to roost during both the breeding season and migration, e.g., Hirundo rustica Linnaeus, 1758, Motacilla alba Linnaeus, 1758, and Sturnus vulgaris Linnaeus, 1758 [53].
The Bearded Reedling, Panurus biarmicus, has a wide range across the Palearctic realm. It is evaluated as Least Concern in the Red List Assessment [7]. The distribution of the species has seen significant gains elsewhere across its European range [26], but the populations in Slovakia have decreased within the last few years [6] and they are considered as Near Threatened there [10].
We focused on the lice of P. biarmicus, and only two species of chewing lice are known from this host – Menacanthus eurysternus (Burmeister, 1848) and Penenirmus visendus (Złotorzycka, 1964) [40]. Menacanthus lice were first reported from P. biarmicus by Balát [4] who described them as Menacanthus brelihi Balát, 1981. Balát [4] included one picture of male parameres and photographs of the holotype female and paratype male, with a short general text description. Consequently, Krištofík [29] examined the Balát’s type material and stated that “examined slides are of bad quality”. He concluded his re-examination of this material with the statement that all the main features of examined specimens were identical to Me. eurysternus. As a result, he synonymized Me. brelihi with Me. eurysternus. Penenirmus visendus was originally described as Panurinirmus visendus by Złotorzycka [55] based on a single female from the Bearded Reedling from Poland. Later, Emerson [11] synonymized Panurinirmus with Penenirmus. To date, no description of the male of this species has been published.
In this paper, we extend the knowledge of ectoparasites of passerine birds occurring in reed bed ecosystems. The aims of this paper are to: (1) present new data on the species distribution of chewing lice found on birds in reed beds in southwestern Slovakia; (2) clarify information on their infestation characteristics; (3) redescribe both sexes of Penenirmus visendus and Menacanthus brelihi and resurrect the latter as a valid taxon; and (4) confirm the validity of these taxa also by phylogenetic analysis of a fragment of the mitochondrial cytochrome oxidase I (COI) gene.
Materials and methods
Birds were captured by mist-netting in reed beds of the National Nature Reserve Parížske močiare located near the villages of Gbelce and Nová Vieska (47°52′ N, 18°30′ E) in southwestern Slovakia. For more details about habitat see Kloubec & Capek [28], about period see Sychra et al. [51]. The fumigation chamber method was applied to collect lice from the birds, using chloroform as a fumigant for 7–10 min, complemented by visual examination of the head. Louse identification was based on papers by Gustafsson & Bush [14], Gustafsson et al. [15], Najer et al. [35], Palma & Price [37], Price [39], Rheinwald [43], Sychra et al. [47, 49–50], and Złotorzycka [56–58]. The taxonomy of birds follows Gill et al. [13].
Measurements were made in QuickPHOTO MICRO 3.1 (Promicra, Prague, Czechia). In the following redescriptions, all measurements are given in millimeters for the following dimensions: ANW = female anus width; AW = abdomen width [at level of segment IV (for Menacanthus) or V (for Penenirmus)]; GSL = male genital sac sclerite length; GW = male genitalia width; HL = head length (at midline); HW = head width (at temples); MW = metathorax width; POW = preocular width; PTW = pterothorax width; PW = prothorax width; TL = total length.
The specimens examined are deposited at the Moravian Museum, Brno, Czechia (MMBC), Museum of Natural History, University of Wrocław, Poland (MNHW), and at the Department of Biology and Wildlife Diseases, University of Veterinary Sciences Brno, Czechia (UVSB).
Infestation characteristics were counted as in Sychra et al. [46, 48]. We used the following categories to designate the infestation on passerine hosts: very light infestation (1–10 lice per bird); light infestation (11–20 lice); medium infestation (21–30 lice); heavy infestation (31–50 lice); very heavy infestation (51–100 lice).
Sequences of a 379 bp fragment of the COI gene were obtained from Menacanthus brelihi and from Penenirmus visendus from Panurus biarmicus using methods described by Johnson et al. [22]. New sequences (GenBank accession numbers OR533291–OR533294, OR626644–OR626646) were aligned together with all available sequences from Menacanthus and Penenirmus genera previously published in the literature and deposited in GenBank [21–24, 31–32, 34, 49, 52] using Geneious 9.1.8 [25] in order to assess their genetic divergence and interspecific relationships. For phylogenetic analysis, we first computed the Akaike information criterion (AIC) computed in MEGA 7.0.14 [30] to identify the most appropriate model of nucleotide substitution. The phylogenetic tree was built with the Bayesian inference analysis (BI) using the Mr. Bayes 3.2.6 plugin in Geneious 9.1.8 [25, 44] with a GTR + G + I model for 10(7) generations, with trees sampled every 1,000 generations. A majority rule consensus tree was summarized after discarding 1,000 trees as a burn-in. Computation of genetic p-distances was performed in MEGA 7.0.14 [30].
Results
A total of 1,621 wild birds representing 34 species were examined for chewing lice (Table 1). A total of 377 (23.3%) birds representing 15 species were parasitized by 26 species of chewing lice (Table 1). A total of 33 louse-host associations were found, which represented more than 1/2 of the known louse-host associations (n = 56) for these 26 bird species examined within their range of distribution [36, 40]. Most birds, i.e., 341 (90.5%, n = 377), showed only very light (1–10 lice/host; 76.7%) to light infestations (11–20 lice/host; 13.8%). Medium (21–30 lice/host) and heavy infestation (31–40 lice/host) were recorded on 14 (3.7%) and 16 (4.2%) birds, respectively. The highest infestations were found on two Hirundo rustica parasitized by 107 and 66 individuals of Acronirmus gracilis (Burmeister, 1838), two Panurus biarmicus parasitized by 71 and 61 individuals of Penenirmus visendus, one Acrocephalus schoenobaenus (Linnaeus, 1758) parasitized by 64 individuals of Menacanthus curuccae (Schrank, 1776), and one Sturnus vulgaris parasitized by 51 individuals of Brueelia nebulosa (Burmeister, 1838). Five of these birds were examined between April 13 and May 1, while one P. biarmicus was examined in October.
List of hosts and their chewing lice. P/E = prevalence = number of birds parasitized (P)/number of birds examined (E); MI = mean intensity = number of individuals of a particular chewing louse species on infested hosts; MA = mean abundance = number of individuals of a particular chewing louse species on examined birds; Σ = a total number of collected lice; N = nymphs; M = Menoponidae; P = Philopteridae; R = Ricinidae.
The majority of birds, i.e., 332 (88%, n = 377), were parasitized by only one species of chewing louse; the co-occurrence of two species of lice was recorded from only 43 birds. In 38 cases, co-occurrence of one ischnoceran and one amblyceran louse species was found, in four cases two species of ischnoceran lice were recorded, and in only one case co-inhabitance of two species of amblyceran lice was recorded (Table 1). Co-occurrence of three species was recorded only on two Sturnus vulgaris. Almost all species of chewing lice were found only on one host species, with the exception of Me. curuccae, Philopterus citrinellae (Schrank, 1776), and Philopterus microsomaticus Tandan, 1955, which were recorded on two species of birds (Table 1). The proportion of individuals belonging to twelve genera of lice is ranked as follows: Penenirmus (32.6%), Menacanthus (29.4%), Brueelia (12.6%), Acronirmus (10.8%), Philopterus (7.7%), Myrsidea (4.2%), Rallicola (1.7%), Sturnidoecus (0.5%), Machaerilaemus (0.2%), Ricinus (0.1%), Fulicoffula (0.1%), and Rostrinirmus (0.04%, n = 2420). Here we did not include data about 706 Penenirmus visendus and 25 Menacanthus brelihi from Bearded Reedlings collected in 2019, when collections were focused only on lice from this host.
Chewing lice of the Bearded Reedling (Panurus biarmicus)
Redescriptions
Order: Psocodea Hennig, 1966
Suborder: Troctomorpha Roesler, 1944
Infraorder: Phthiraptera Haeckel, 1896
Parvorder: Amblycera Kellogg, 1896
Family: Menoponidae Mjöberg, 1910
Genus: Menacanthus Neumann, 1912
Menacanthus brelihi Balát, 1981
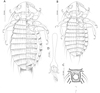 |
Figure 1 Menacanthus brelihi Balát, 1981. (A) Female dorso-ventral view; (B) Male dorso-ventral view; (C) Detail of gula; (D) Male genitalia, dorsal view. |
 |
Figure 2 Menacanthus brelihi Balát, 1981. (A) Holotype female; (B) Allotype male; (C) Type slides; (D) Non-type female. |
Menacanthus brelihi Balát, 1981: 273: fig. 1, Plate I, Figures 1–2 [4].
Type host: Panurus biarmicus (Linnaeus, 1758) – Bearded Reedling (Panuridae).
Type locality: Velký Dvůr near Pohořelice (south Moravia), Czechia
Remarks. Menacanthus brelihi belongs to the curuccae species group (sensu Martinů et al. [32]). Both sexes of this species are readily identified by combination of characters as follows: (1) each side of metanotum with 3–5 lateroanterior setae; (2) characteristic shape of gular plate, with large central lighter “hole” and several small anterior ones, posterior margin straight or undulated, all four setae on each side are inserted in clear lateral area; (3) ocular seta 19 finer than outer central pronotal seta 1; (4) pleurites with anterior setae; (5) large number of sternal setae, especially on sternites III–VI of females (each with more than 53 setae) and sternites III–V of males (each with more than 34 setae); and (6) quite large size. In the key by Price [39], the female of Me. brelihi would key out to couplet 28, being closest to Me. robustus (Kellogg, 1896). However, the female of Me. brelihi can be distinguished from that of Me. robustus by a different number of setae on tergites VII and IX (28–29 and 23–26 vs. 23–26 and 32–33) and sternites IV–V (67–72 and 63–75 vs. 60–65 and 51–56). Although males of Me. robustus are unknown, the male of Me. brelihi would key out to couplet 35 in the key by Price [39], being closest to Me. tenuifrons Blagoveshtchensky, 1940. However, the male of Me. brelihi can be distinguished from that of Me. tenuifrons by a different number of setae on tergite IX (7–8 vs. 10–11) and sternites IV–VII (a total of 114–143 vs. 97–118).
In order to add Me. brelihi to Price’s [39] key, the following alterations should be made:
28. Temple width at least 0.57, metathorax width at least 0.56; both anal fringes of over 50 setae; ventral spinous head process at least 0.08 long ......................28a
– Temple width not over 0.56, metathorax width not over 0.55; either or both anal fringes of under 50 setae; ventral spinous head process shorter as long as above ….........29
And insert new couplet among couplets 28 and 29 as follows:
28a. Tergite VII with at least 28 setae, tergite IX with not more than 26 setae; sternite IV with at least 67 setae, sternite V with at least 63 setae …Me. brelihi
– Tergite VII with not more than 26 setae, tergite IX with at least 32 setae; sternite IV with not more than 65 setae, sternite V with not more than 56 setae …Me. robustus
35. Sternites III–IV each with over 30 setae; subgenital plate with at least 14 setae ................35a
– Sternites III–IV each with under 30 setae; subgenital plate with only up to 12 setae …Me. sinuatus
And insert new couplet among couplets 35 and 36 as follows:
35a. Tergite IX with not more than 8 setae and sternites IV–VII with a total of 114–143 setae …Me. brelihi
– Tergite IX with at least 10 setae and sternites IV–VII with a total of 97–118 setae …Me. tenuifrons
A fragment of the COI gene was sequenced from three specimens of Me. brelihi (GenBank accession numbers OR626644–OR626646). Compared to all available sequences of the Menacanthus genus, our sequences clustered together with lice of Me. takayamai with a sequence divergence of 14–16%. These sequence divergences are large enough to confirm Me. brelihi as a separate species. Phylogenetic relationships among sequences obtained from Me. brelihi and sequences from other Menacanthus species are presented in Figure 3 and Supplementary Figure S1.
 |
Figure 3 Diagram of the evolution of the Menacanthus lineages. The topology was adapted from the cytochrome oxidase subunit I phylogeny in Supplementary Figure S1. The origin of the samples is in parentheses: AF – Afrotropical realm; EPA – Eastern Palearctic realm; NA – Nearctic realm; NT – Neotropical realm; OR – Oriental realm; WPA – Western Palearctic realm. |
Female (n = 7). As in Figures 1A and 2A. Head with rounded anterior margin, preocular slit, hypopharyngeal sclerites weakly developed and ventral spinous process 0.08–0.09 mm long. Gular plate pigmented, with large central lighter “hole” and several small anterior ones, posterior margin straight or undulated, all four setae on each side are inserted in clear lateral area (Figs. 1C, 2D). Long occipital setae 21, 22, and 23, with alveoli in straight line. Head seta 24 0.20–0.25 mm long. Ocular seta 19 fine 0.02–0.03 mm long. Outer central pronotal setae short and stout. Pronotal margin with 10 long and 4 short setae; prosternal plate moderately developed. Metanotum with 3–5 anterolateral setae on each side and 15–16 marginal setae; mesosternal plate with 10–14 setae; metasternal plate with 12–14 setae. Tergal setae: I, 22–24; II, 24–30, III, 28–31, IV, 26–31, V, 30–34, VI, 28–33; VII, 28–29; VIII, 17–19; IX, 23–26. Pleurites III–VI with 3–5 anterior setae. Sternal setae: I, 3–5; II, 38–46; III, 56–69; IV, 67–72; V, 63–75; VI, 53–63; VII, 41–53; subgenital plate, 25–29, vulval margin with 20–23 setae and prominent deep serrations medioposteriorly. With medioanterior setae on sternites II–VII. Only one very long on each side of posterior margin of abdomen extending beyond ends of anal fringe setae. Ventral and dorsal anal fringes of 57–60 setae.
Dimensions (data for the holotype are in parentheses): POW = 0.47–0.51 (0.50); HW = 0.63–0.65 (0.66); PW = 0.44–0.49 (0.49); MW = 0.59–0.66 (0.64); AW = 0.83–0.93 (0.84); ANW = 0.28–0.31 (0.29); TL = 1.79–2.07 (1.94).
Male (n = 6). As in Figures 1B and 2B similar to female. Head seta 24 0.17–0.18 mm long. Metanotum with 8–12 marginal setae; mesosternal plate with 8–10 setae; metasternal plate with 10–14 setae. Tergal setae: I, 13–15; II, 17–21; III, 20–22; IV, 19–22; V, 18–21; VI, 17–22; VII, 15–18; VIII, 10–11; IX, 7–8. Pleurites III–VI with 1–2 anterior setae. Sternal setae: I, 2–3; II, 26–38; III, 36–52; IV, 39–47; V, 34–40; VI, 25–33; VII, 16–23; VIII, 8–12; subgenital plate, 6–7 plus 10–11 on posterior margin. Genitalia as on Figure 1D.
Dimensions (data for the allotype are in parentheses): POW = 0.42–0.44 (0.45); HW = 0.54–0.57 (0.56); PW = 0.37–0.39 (0.39); MW = 0.45–0.48 (0.52); AW = 0.59–0.62 (0.66); GW = 0.08–0.10 (0.12); TL = 1.40–1.47 (1.56).
Examined material. Holotype ♀ ex Panurus biarmicus, Velký Dvůr near Pohořelice (south Moravia), Czechia, 15 Jun. 1961, Balát’s collection no. FB1394 (MMBC, Fig. 2C). Paratypes: 1♂ (FB1393, noted as Allotype), 1♀ (FB1395), same data as holotype; 1♀ (FB1225) Nová Ves near Pohořelice (south Moravia), Czechia, 18 Jul. 1962; 3♀♀ from the same host, Neusiedl am See, Austria, 17–18 Sep. 1960 (FB1230, 1234, 1235), leg. Balát (MMBC).
Other material. Non-types ex P. biarmicus: 4♀♀, 4♂♂, Gbelce, Slovakia, 16–18 Jul. 2019, leg. Sychra & Ošlejšková (UVSB).
Note: Balát’s collection at MMBC includes seven slides with Me. brelihi. According to Balát’s notes, all three specimens from the type series were collected from an adult male of P. biarmicus. Although Balát [4] mentioned a total of 11 lice (1♂, 3♀♀ and 7 nymphs) from the adult female of the type host that were examined at Nová Ves, only one slide with one female and one nymph is present at the MMBC. Similarly, according to Balát’s notes, specimens from Austria were collected from two females (2♀♀ from 17 Sep. 1960) and one male of P. biarmicus (1♂, 4♀♀ and 2 nymphs from 18 Sep. 1960), but only three females had been mounted on the slide, while other specimens were stored in ethanol. Except for the specimens mentioned within the examined material, all other specimens are missing from the MMBC and must be regarded as lost.
Parvorder: Ischnocera Kellogg, 1896
Family: Philopteridae Burmeister, 1838
Genus: Penenirmus Clay & Meinertzhagen, 1938
Penenirmus visendus (Złotorzycka, 1964)
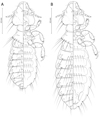 |
Figure 4 Penenirmus visendus (Złotorzycka, 1964). (A) Male dorso-ventral view; (B) Female dorso-ventral view. |
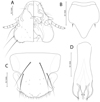 |
Figure 5 Penenirmus visendus (Złotorzycka, 1964). (A) Male head dorso-ventral view; (B) Dorsal anterior head plate; (C) Female subgenital plate and vulval margin, ventral view; (D), Male genitalia dorsal view. |
 |
Figure 6 Penenirmus visendus (Złotorzycka, 1964). (A) Holotype female; (B) Holotype slide; (C) Non-type Female; (D) Non-type male. |
Panurinirmus visendus Złotorzycka, 1964: 270: fig. 8d, photo 15 [55].
Penenirmus visendus Emerson, 1972: 111 [11].
Penenirmus visendus Price, et al. [in Price et al.], 2003 [40].
Type host: Panurus biarmicus (Linnaeus, 1758) – Bearded Reedling (Panuridae).
Type locality: Górki Wschodnie near Gdańsk, Poland
Remarks. Złotorzycka [55] provided a very poor description of P. visendus under the name Panurinirmus visendus based on a single female from P. biarmicus from Poland. There is no comprehensive morphological revision of Penenirmus from passerine birds and that is one reason why species determination is difficult and a common practice is to identify species based on host records. To date, 11–14 species of Penenirmus have been reported from Europe [33, 40, 57]. To partially address the difficulties in identifying lice in this genus, we compare P. visendus with P. albiventris a recently well-described by Sychra et al. [49]. We found only limited morphological differences between these two species concerning mainly slight differences in the shape of the dorsal anterior head plate (with short blunt posterior process in P. visendus compared with quite long and pointed process in P. albiventris) and abdominal tergites II–VI (joined by two narrow conspicuously pigmented strips compared with tergites that are joined by a single pigmented strip).
A fragment of the COI gene was sequenced from four specimens of P. visendus (GenBank accession numbers OR533291–OR533292). Comparing our sequences with other available sequences from Penenirmus genus, the closest were those of P. albiventris, with sequence divergences around 18–19%. These sequence divergences are large enough to confirm P. visendus as a separate species, at least until sequences from other species are known. These data support the aforementioned morphological differences. Phylogenetic relationships among sequences obtained from P. visendus and sequences from other Penenirmus species are presented in Figure 7.
 |
Figure 7 Phylogenetic tree of the Penenirmus species based on partial COI sequences, estimated with Bayesian analysis based on a 379 bp alignment of a COI gene fragment. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The numbers above the branches indicate Bayesian posterior probabilities. Branches with posterior probabilities < 0.5 were collapsed. New sequences are in bold type. The origin of the samples is in parentheses: AF – Afrotropical realm; NA – Nearctic realm; NT – Neotropical realm; OR – Oriental realm; WPA – Western Palearctic realm. |
Male (n = 20). As in Figures 4A and 6D. Head (Fig. 5A) with postantennal suture, with one post-nodal and three post-temporal setae on each side, all of them short and spine-like. Marginal temporal setae 1 and 3 long, other marginal temporal setae short. Anterior dorsal setae of forehead shorter than distance between them. Dorsal anterior head plate quite large with slightly concave anterior margin and short blunt posterior process (Fig. 5B). Metanotum and metapleurite with an almost continuous row of 7 evenly spaced setae on each side (outmost lateral short metapleural seta included). Mesosternal plate with 2 setae, metasternal plate with 4 setae.
Tergites II–VI with anterior median notches, joined by two narrow conspicuously pigmented strips. Postspiracular setae on tergites III–VII long (0.28–0.33). Posterocentral tergal setae: II, 5–6; III, 6–7; IV, 6–7; V, 6–7; VI, 5–7; VII, 2–4; VIII, 2; IX, 4–6. Sternites lightly sclerotized with almost inconspicuous lateral plates. Sternal setae: II, 5–6; III, 9–12; IV, 10–12; V, 8–10; VI, 7–8; VII, 2. Paratergal setae: II–III, 0; IV–V, 1; VI–VII, 2; VIII–IX, 3. Genitalia as in Figure 5D.
Dimensions: HL = 0.38–0.49; POW = 0.29–0.37; HW = 0.35–0.48; PW = 0.22–0.31; PTW = 0.31–0.48; AW = 0.40–0.68; GW = 0.07–0.10; TL = 1.50–2.05.
Female (n = 20). As in Figures 4B, 6A and 6C. As for male, except as follows: head with only one short spine-like post-temporal setae on each side.
Tergites II–VIII with anterior median notches. Postspiracular setae 0.31–0.37 long. Posterocentral tergal setae: II, 6–8; III, 5–8; IV, 7–10; V, 6–9; VI, 6–8; VII, 6–7; VIII, 4; IX, 2. Sternal setae: II, 6; III, 7–10; IV, 8–11; V, 8–9; VI, 7–9; VII, 2; VIII, 2. Subvulval sclerites well-developed. Subgenital plate wide and slightly convex posteriorly, with 25–30 fine and 8–10 very short spine-like setae (Fig. 5C).
Dimensions (data for the holotype are in parentheses): HL = 0.42–0.51 (0.45); POW = 0.32–0.41 (0.33); HW = 0.41–0.52 (0.44); PW = 0.24–0.33 (0.24); PTW = 0.35–0.56 (0.42); AW = 0.54–0.92 (distorted); TL = 1.86–2.05 (1.95).
Examined material. Holotype ♀ ex Panurus biarmicus, Górki Wschodnie near Gdańsk, Poland, no. 9/c/1, leg. Zajac (MNHW No 786, Fig. 6B).
Other material. Non-types ex P. biarmicus: 5♂♂, 22♀♀ and 1 nymph, Velký Dvůr near Pohořelice (south Moravia), Czechia, 15 Jun. 1961, Balát’s collection no. FB1226, 1227, 1228, 1392 (MMBC); 2♂♂ and 2 nymphs from the same host, Neusiedl am See, Austria, 17 and 18 Sep. 1960 (FB1231, 1236), leg. Balát (MMBC); 20♂♂, 20♀♀, Gbelce, Slovakia, 20–30 Apr. 2009, 16–18 Jul. 2019, leg. Sychra & Ošlejšková (UVSB).
Note: According to Balát’s notes, there should be six items with P. visendus in his collection at the MMBC, under the numbers FB1226–28, 1231, 1236, and 1392. A total of 69 P. visendus had been collected from two males (single ♀ as item no. 1392 and 3♂♂, 12♀♀ and 14 nymphs as item no. 1228) and one female of P. biarmicus (6♂♂, 9♀♀ and 24 nymphs as item nos. 1226–27) examined on 15 June 1961 at Velký Dvůr near Pohořelice (south Moravia), Czechia. Additional 2♂♂ (FB1231) and two nymphs (FB1236) had been collected from male (examined on 18 Sep. 1960) and female of P. biarmicus (examined on 17 Sep. 1960) in Neusiedl am See, Austria, respectively. Balát considered these lice a new species, because he noted that item nos. 1226 and 1227 contain the holotype and paratype, respectively. However, he never formally described them. Despite Balát’s notes, not all aforementioned lice were mounted on slides, so at present there is a total of 7♂♂, 22♀♀ and 3 nymphs of P. visendus on 19 slides with numbers FB1226 (1 slide), 1227 (6 slides), 1228 (9 slides), 1231 (1 slide), 1236 (1 slide), and 1392 (1 slide) deposited at the MMBC. All other specimens are missing from the MMBC and must be regarded as lost.
Discussion
The avifauna of the reed beds represents a mixture community of both habitat specialists that breed in this environment, as well as bird species that only roost in reed stands during both the breeding season and migration. In the case of habitat specialists, Sychra et al. [51] found significantly higher prevalences and mean abundances of chewing lice on resident and short-distance migrants (Acrocephalus melanopogon, Panurus biarmicus) than on long-distance migratory species (Acrocephalus scirpaceus, A. schoenobaenus, Locustella luscinioides). Except for the aforementioned birds, the highest infestations of lice in this study were found on birds roosting in reed beds in larger flocks, such as Hirundo rustica or Sturnus vulgaris. Horizontal transmission of lice is more frequent in communal roosting places where close contact between larger number of individuals can take place. Prevalence and intensity of lice are thus usually higher as well on these birds [27, 45]. We suggest that reed beds play an important role in the maintenance and dispersal of ectoparasites in the population of these hosts. On the other hand, despite large sample sizes, neither lice nor eggs were found on Acrocephalus arundinaceus (n = 107), even though Menacanthus curuccae and Philopterus fedorenkoae (Mey, 1983) are known from this host [35, 40].
In the present study, we evaluated 33 host-louse associations including 12 genera of lice. These associations include both 1) host-generalist, parasitizing more than one host species and host-specific lice, occurring only on single host species, and 2) lice species with broad geographic distribution, reported across the range of distribution of their hosts and lice species with only rare records in a limited area (for hosts see Price et al. [40]; for distribution see Mey [33], unless otherwise noted).
Among amblyceran lice, we found mainly host-generalist members of three genera that were also well recorded across Europe: Menacanthus – Me. eurysternus (reported from more than 170 hosts all around the world) [32], Me. curuccae and Me. sinuatus (Burmeister, 1838) (13 and 8 hosts, respectively in the Palearctic and Nearctic realms); Myrsidea – My. cucullaris (Nitzsch, 1818) (2 hosts in the Palearctic realm), My. rustica (3 hosts all around the world), and My. quadrifasciata (Piaget, 1880) (33 hosts all around the world) [50]; and Ricinus – R. fringillae De Geer, 1778 (47 hosts all around the world) [43].
An example of a widely distributed host-specific louse species is Myrsidea latifrons (Carriker [& Shull], 1910) that was reported from its host Riparia riparia (Linnaeus, 1758) across the world, including most places in Europe (Kolenčík & Sychra, unpublished data).
Among rare species, we can name one host-generalist: Menacanthus chrysophaeus (Kellogg, 1896), and three host-specific species: Machaerilaemus clayae (Balát, 1966), Menacanthus brelihi Balát, 1981 , and Menacanthus obrteli Balát, 1981.
Menacanthus chrysophaeus was originally described from six hosts in the Nearctic realm [39], but recently it was also reported from Emberiza schoeniclus (Linnaeus, 1758) from Turkey, Greece, and Spain [5].
Machaerilaemus clayae is another parasite of Riparia riparia, but contrary to Myrsidea latifrons there are only a few records of this species from Czechia [3], Romania [1], Moldova, and Russia (Volga-Kama Nature Reserve) [12].
Menacanthus obrteli was originally described by Balát [4], but this name was later synonymized with Me. takayamai Uchida, 1926 [40]. Nevertheless, Sychra et al. [47] recently confirmed Me. obrteli as a valid species with Locustella luscinioides as the only host (see also Martinů et al. [32]). To date, there are only a few records of this louse from Czechia [4, 47], Slovakia [36], and Hungary [54].
We can see the same scenario for Menacanthus brelihi from P. biarmicus. This species was originally described by Balát [4]. Consequently, Krištofík [29] synonymized Me. brelihi with Me. eurysternus. After examination of type material and newly collected material we can confirm Balát′s assertion. Analysis of the COI gene shows that Me. brelihi is close to Me. takayamai, but the high divergence of sequences of these two species (14–16%) confirms Me. brelihi is a separate species. Moreover Me. takayamai seems to be paraphyletic and additional molecular analyses including more genes are needed to confirm relationships within this complex of species. To date, Me. brelihi has been reported from P. biarmicus from Czechia and Austria [4], and from Romania [42], and based on our sampling also from Slovakia [36, 51].
In the case of ischnoceran lice, we found several host-generalists that are also well recorded across Europe: Acronirmus gracilis (reported from 12 hosts all around the world) [14], Penenirmus auritus (Scopoli, 1763) (52 hosts all around the world), Penenirmus albiventris (2 hosts in the Palearctic, Nearctic, and Neotropical realms) [49], Philopterus citrinellae (Schrank, 1776) (16 hosts in the Palearctic realm) [37], Rostrinirmus ruficeps (Nitzsch [in Giebel], 1866) (5 hosts in the Palearctic, Oriental, and Afrotropical realms) [40].
Examples of widely distributed host-specific lice are Brueelia nebulosa (Burmeister, 1838) and Sturnidoecus sturni (Schrank, 1776) that are reported on the host Sturnus vulgaris from most areas of Europe, and Rallicola cuspidatus (Scopoli, 1763) as a specific parasite of Rallus aquaticus.
Among rare species, we can name two host-generalists: Philopterus acrocephalus Carriker, 1949 (5 hosts in the Palearctic realm) [35], and Philopterus microsomaticus Tandan, 1955 (3 hosts; reported in Europe only from Finland and Poland); and four host-specific species: Brueelia blagovescenskyi Balát, 1955 (in Europe reported from Czechia, Germany, Hungary, and Spain), Brueelia locustellae Fedorenko, 1975 (in Europe reported from Germany and Ukraine), Brueelia vaneki Balát, 1981 (reported only rarely from Czechia and Slovenia) [14, 15], and Penenirmus visendus. To date, P. visendus has been reported only from Poland [55] and Romania [42], and based on our sampling also from Slovakia [36, 51]. Undetermined species of Penenirmus have also been reported from Hungary by Rékási [41]. We extend the area of distribution of this louse species to include Austria and Czechia.
In our study, Panurus biarmicus has fragmented distributions in Central Europe. Bush et al. [9] showed that habitat fragmentation may impact the prevalence of lice. The low infestation indices of Menacanthus brelihi may follow a similar scenario, i.e., smaller populations of hosts on the edge of their range may harbor fewer lice [38]. Sampling of P. biarmicus in other parts of its range is necessary to confirm that the rareness of this species is related to habitat fragmentation.
On the other hand, our results show that Penenirmus visendus is a common parasite of P. biarmicus, occurring with quite high prevalence throughout year [51]. Therefore, we suppose that P. visendus could have a stronger impact on its host than Me. brelihi. However, we cannot exclude that P. visendus is, on the contrary, a less virulent parasite that can occur in higher numbers without having a significant effect on the condition of its host. An experimental study is necessary to evaluate these assumptions. The present study also demonstrated the importance of accurate identification of parasites, especially on rarely examined and endangered species, where the knowledge of parasite diversity can be useful in their conservation programs. Moreover, Gustafsson et al. [16] suggested that parasites on endangered hosts, especially those that are host-specific, should also be treated as endangered. Panurus biarmicus is evaluated as Near Threatened in Slovakia [10], so if we adopt the idea of Gustafsson et al. [16], then both louse species on this host, but especially Me. brelihi, may be considered to have the same conservation status.
Acknowledgments
We thank all our co-workers in the field. We especially thank Igor Malenovský (Moravian Museum Brno, Czechia) and Marek Wanat (Museum of Natural History, University of Wrocław, Poland) for enabling us to examine the type specimens from Balát’s and Złotorzycka’s collections, respectively. We would also like to thank the two anonymous reviewers for their help in improving the published version of this manuscript. We were supported by the project 2023ITA22 from the University of Veterinary Sciences, Brno, Czechia.
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary material
 |
Supplementary Figure S1 Phylogenetic tree of the Menacanthus species based on partial COI sequences, estimated with Bayesian analysis based on a 379 bp alignment of a COI gene fragment. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The numbers above the branches indicate Bayesian posterior probabilities. New sequences are in bold type. — Colors: purple – samples from the Western Palearctic realm; dark purple – samples from the Eastern Palearctic realm; red – samples from the Afrotropical realm; green – samples from the Oriental realm; light blue – samples from the Nearctic realm; dark blue – samples from the Neotropical realm. |
References
- Adam C. 2007. Data on the chewing louse fauna (Phthiraptera: Amblycera, Ischnocera) from some Romanian autochthonous and exotic birds. Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa”, 50, 145–210. [Google Scholar]
- Balát F. 1955. Chewing lice from Tatra national park. Folia Zoologica et Entomologica, 4(4), 389–398. (In Czech). [Google Scholar]
- Balát F. 1966. Federlinge tschechoslowakischer Uferschwalben. Angewandte Parasitologie, 7, 244–248. [Google Scholar]
- Balát F. 1981. A contribution to the knowledge of biting lice (Mallophaga) found on passerines (Passeriformes). Folia Parasitologica, 28, 273–282. [Google Scholar]
- Bernal I, Talabante C, Dik B, Sánchez Martínez LJ, Viejo JL. 2022. Low prevalence of chewing lice (Phthiraptera) in wintering populations of the reed bunting Emberiza schoeniclus (Aves: Passeriformes: Emberizidae) in the Iberian Peninsula. Annals of Parasitology, 68(1), 177–181. [PubMed] [Google Scholar]
- Birds Directive. 2023. Panurus biarmicus – detailed species summary: Population status and trends at the EU and Member State levels under Article 12 web tool for the period 2013–2018. (accessed 2 September 2023). https://nature-art12.eionet.europa.eu/article12/summary?period=3&subject=Panurus+biarmicus&reported_name=. [Google Scholar]
- BirdLife Internatinal 2023. Species factsheet: Panurus biarmicus. (accessed 2 September 2023). http://datazone.birdlife.org/species/factsheet/bearded-reedling-panurus-biarmicus. [Google Scholar]
- Bush SE, Gustafsson DR, Clayton DH. 2018. New records of ectoparasites from passerine birds in the High Tatras of Slovakia. Oecologia Montana, 27, 43–45. [Google Scholar]
- Bush SE, Reed M, Maher S. 2013. Impact of forest size on parasite biodiversity: implications for conservation of hosts and parasites. Biodiversity and Conservation, 22, 1391–1404. [CrossRef] [Google Scholar]
- Demko M, Krištín A, Puchala P. 2013. Red list of birds in Slovakia. Tichodroma, 225, 69–78. [Google Scholar]
- Emerson KC. 1972. Checklist of the Mallophaga of North America (north of Mexico). Part I. Suborder Ischnocera. , Dugway, Utah: . Deseret Test Center, Dugway Proving Ground. p. 200. [Google Scholar]
- Fedorenko IA. 1983. Superfamily Menoponoidea, Fauna Ukrainy 22(5). Institut Zoologii Akademii Nauk Ukraini: RSR, Kiev. p. 1–168. (in Russian). [Google Scholar]
- Gill F, Donsker D, Rasmussen P, Editors. 2023. IOC World Bird List (v13.2). (Accessed 2 September 2023). https//doi.org/10.14344/IOC.ML.13.2. [Google Scholar]
- Gustafsson DR, Bush SE. 2017. Morphological revision of the hyperdiverse Brueelia-complex (Insecta: Phthiraptera: Ischnocera: Philopteridae) with new taxa, checklists and generic key. Zootaxa, 4313, 1–443. [CrossRef] [Google Scholar]
- Gustafsson DR, Ošlejšková L, Najer T, Sychra O, Zou F. 2019. Redescriptions of thirteen species of chewing lice in the Brueelia-complex (Phthiraptera, Ischnocera, Philopteridae), with one new synonymy and a neotype designation for Nirmus lais Giebel, 1874. Deutsche Entomologische Zeitschrift (neue Folge), 66, 17–39. [CrossRef] [Google Scholar]
- Gustafsson DR, Tian C, Yu X, Xu L, Wu S, Zou F. 2021. Unintentional parasite conservation success: chewing lice recovered from crested ibis, Nipponia nippon, in breeding program facilities in Shaanxi, China. Biodiversity and Conservation, 30, 3939–3963. [CrossRef] [Google Scholar]
- Janiga M. 2018. Different coevolutionary breeding strategies of Ischnoceran lice on Prunella collaris and P. modularis in high mountains. Polish Journal of Ecology, 66(2), 182–193. [CrossRef] [Google Scholar]
- Janiga M. 2019. Adaptive plasticity in insect parasites – Philopterus lice and their accentor passerine hosts. Polish Journal of Ecology, 66(4), 395–406. [CrossRef] [Google Scholar]
- Janiga M, Kubašková Ľ. 2000. The biology of the alpine accentor Prunella collaris. III. The coevolution of alpine accentors and lice (Phthiraptera). Oecologia. Montana, 9(1–2), 24–28. [Google Scholar]
- Janiga M, Mičková A. 2004. The biology of the alpine accentor Prunella collaris. V. The sex ratio and transmission of lice Philopterus emiliae. Oecologia Montana, 13, 17–22. [Google Scholar]
- Johnson KP, Moyle RG, Witt CC, Faucett RC, Weckstein JD. 2001. Phylogenetic relationships in the louse genus Penenirmus based on nuclear (EF-1alpha) and mitochondrial (COI) DNA sequences. Systematic Entomology, 26(4), 491–497. [CrossRef] [Google Scholar]
- Johnson KP, Adams RJ, Clayton DH. 2002. The phylogeny of the louse genus Brueelia does not reflect host Phylogeny. Biological Journal of the Linnean Society London, 77(22), 233–247. [CrossRef] [Google Scholar]
- Johnson KP, Weckstein JD, Witt CC, Faucett RC, Moyle RG. 2002. The perils of using host relationships in parasite taxonomy: phylogeny of the Degeeriella complex. Molecular Phylogenetics and Evolution, 23(2), 150–157. [CrossRef] [PubMed] [Google Scholar]
- Johnson KP, Cruickshank RH, Adams RJ, Smith VS, Page RD, Clayton DH. 2003. Dramatically elevated rate of mitochondrial substitution in lice (Insecta: Phthiraptera). Molecular Phylogenetics and Evolution, 26(2), 231–242. [CrossRef] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [CrossRef] [PubMed] [Google Scholar]
- Keller V, Herrando S, Voříšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvaňová A, Kalyakin MV, Bauer HG, Foppen RPB. 2020. European Breeding Bird Atlas 2: Distribution. Barcelona: Abundance and Change. European Bird Census Council & Lynx Edicions. [Google Scholar]
- Kettle PR. 1983. The seasonal incidence of parasitism by Phthiraptera on starlings (Sturnus vulgaris) in England. New Zealand Entomologist, 7(4), 403–408. [CrossRef] [Google Scholar]
- Kloubec B, Capek M. 2005. Seasonal and diel budgets of song: a study of Savi’s warbler (Locustella luscinioides). Journal of Ornithology, 146, 206–2014. [CrossRef] [Google Scholar]
- Krištofík J. 2000. Synonymical notes to the Menacanthus species (Phthiraptera, Menoponidae) living on Passeriformes (Aves). Acta Parasitologica, 45(1), 57–58. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Phylogenetics and Evolution, 33, 1870–1874. [Google Scholar]
- Light JE, Nessner CE, Gustafsson DR, Wise SR, Voelker G. 2016. Remarkable levels of avian louse (Insecta: Phthiraptera) diversity in the Congo Basin. Zoologica Scripta, 45, 538–551. [CrossRef] [Google Scholar]
- Martinů J, Sychra O, Literák I, Capek M, Gustafsson DR, Štefka J. 2015. Host generalists and specialists emerging side by side: an analysis of evolutionary patterns in the cosmopolitan chewing louse genus Menacanthus . International Journal for Parasitology, 45, 63–73. [CrossRef] [PubMed] [Google Scholar]
- Mey E. 2023. Phthiraptera, in de Jong Y, Editor. Fauna European Consortium. Checklist dataset. (accessed 23 August 2023).. https://doi.org/10.15468/ymk1bx. [Google Scholar]
- Najer T, Sychra O, Kounek F, Papoušek I, Nguyen MH. 2014. Chewing lice (Phthiraptera: Amblycera, Ischnocera) from wild birds in southern Vietnam, with descriptions of three new species. Zootaxa, 3755(5), 419–433. [CrossRef] [PubMed] [Google Scholar]
- Najer T, Papoušek I, Adam C, Trnka A, Quach VT, Nguyen CN, Figura R, Literák I, Sychra O. 2020. New records of Philopterus (Ischnocera: Philopteridae) from Acrocephalidae and Locustellidae, with description of one new species from Regulidae. European Journal of Taxonomy, 632, 1–37. [Google Scholar]
- Ošlejšková L, Krištofík J, Trnka A, Sychra O. 2021. An annotated checklist of chewing lice (Phthiraptera: Amblycera, Ischnocera) from Slovakia. Zootaxa, 5069, 1–80. [CrossRef] [PubMed] [Google Scholar]
- Palma RL, Price RD. 2006. Lice of the genus Philopterus Nitzsch (Phthiraptera: Ischnocera: Philopteridae) parasitic on host of the genus Emberiza (Passeriformes: Emberizidae). New Zealand Journal of Zoology, 33, 1–6. [CrossRef] [Google Scholar]
- Paterson A, Palma RL, Gray RD. 1999. How frequently do avian lice miss the boat? Implications for coevolutionary studies Systematic Biology, 48, 214–223. [CrossRef] [Google Scholar]
- Price RD. 1977. The Menacanthus (Mallophaga: Menoponidae) of the Passeriformes (Aves). Journal of Medical Entomology, 14, 207–220. [CrossRef] [PubMed] [Google Scholar]
- Price RD, Hellenthal RA, Palma RL, Johnson KP, Clayton DH. 2003. The chewing lice: world checklist and biological overview, The chewing lice: world checklist and biological overview. Illinois Natural History Survey Special Publication. p. 24. [Google Scholar]
- Rékási J. 1993. Bird lice (Mallophaga) parasiting the birds of Hungary. Aquila, 100, 71–93. [Google Scholar]
- Rékási J, Kiss JB, Sándor AD. 2017. Chewing lice (Phthiraptera: Amblycera, Ischnocera) recorded from birds in the Danube Delta Biosphere Reserve: a literature review with new data. Aquila, 124, 7–33. [Google Scholar]
- Rheinwald G. 1968. Die Mallophagengattung Ricinus De Geer, 1778. Revision der ausseramerikanischen Arten. Mitteilungen aus dem Hamburg Zoologischen Museum Institut, 65, 181–326. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. [CrossRef] [PubMed] [Google Scholar]
- Rózsa L, Rékási J, Reiczigel J. 1996. Relationship of host coloniality to the population ecology of avian lice (Insecta: Phthiraptera). Journal of Animal Ecology, 65, 242–248. [CrossRef] [Google Scholar]
- Sychra O, Literák I, Podzemný P, Benedikt V. 2008. Insect ectoparasites from wild passerine birds in the Czech Republic. Parasite, 15, 599–604. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Sychra O, Sychrová V, Literák I. 2008. Identity of Menacanthus obrteli Balát (Phthiraptera: Menoponidae) from the Savi’s Warbler (Passeriformes: Sylviidae). Biologia (B Zool.), 63(5), 686–688. [CrossRef] [Google Scholar]
- Sychra O, Literák I, Podzemný P, Harmat P, Hrabák R. 2011. Insect ectoparasites on wild birds in the Czech Republic during the pre-breeding period. Parasite, 18, 13–19. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Sychra O, Kounek F, Papoušek I, Capek M, Cárdenas-Callirgos JM, Franco S, Literák I. 2014. Chewing lice (Phthiraptera: Amblycera et Ischnocera) from wrens (Passeriformes: Troglodytidae), with description of a new species of Myrsidea. Acta Entomologica Musei Nationalis Pragae, 54(1), 1–27. [Google Scholar]
- Sychra O, Kolenčík S, Papoušek I, Bilbija B, Literák I. 2021. Myrsidea quadrifasciata (Phthiraptera: Amblycera) – a unique host generalist among highly host-specific chewing lice. Arthropod Systematics & Phylogeny, 79, 379–400. [CrossRef] [Google Scholar]
- Sychra O, Ošlejšková L, Skoupá Ž, Najer T, Literák I, Papoušek I, Trnka A, Capek M. 2023. Chewing lice of passerine birds in reed beds in Slovakia, with a special focus on Panurus biarmicus. Medical and Veterinary Entomology, 37, 300–307. [CrossRef] [PubMed] [Google Scholar]
- Takano OM, Voelker G, Gustafsson DR, Light JE. 2018. Molecular phylogeny and novel host associations of avian chewing lice (Insecta: Phthiraptera) from South Africa. Systematic Entomology, 44, 289–304. [Google Scholar]
- Trnka A, Capek M, Kloubec B. 2003. Vtáky národnej prírodnej rezervácie Parížske močiare [Birds of the National Nature Reserve Parížske močiare Marsh, SW Slovakia]. Bratislava: Veda. p. 161. (in Slovak, with an English summary). [Google Scholar]
- Vas Z, Privigyei C, Prohaszka VJ, Csorgo T, Rózsa L. 2012. New species and host association records for the Hungarian avian louse fauna (Insecta: Phthiraptera). Ornis Hungarica, 20(1), 44–49. [CrossRef] [Google Scholar]
- Złotorzycka J. 1964. Mallophaga parasitizing Passeriformes and Pici II. Brueeliinae. Acta Parasitologica Polonica, 12(24), 239–282. [Google Scholar]
- Złotorzycka J. 1976. Chewing lice – Mallophaga, part 2 Superfamily Menoponoidea. Warszava: Panstwowe Wydawnictwo Naukowe. p. 190. (in Polish). [Google Scholar]
- Złotorzycka J. 1997. Chewing lice (Mallophaga), systematic part: Goniodidae and Philopteridae. Wroclaw: Wydawnictwo Uniwersyteta Wroclawskiego. p. 308. (in Polish). [Google Scholar]
- Złotorzycka J, Modrzejewska M. 2001. Chewing lice (Mallophaga), systematic part: Rallicolidae and Pseudonirmidae. Wroclaw: Wydawnictwo Uniwersyteta Wroclawskiego. p. 180. (in Polish). [Google Scholar]
Cite this article as: Sychra O, Sušilová L, Najer T, Literák I, Papoušek I, Martinů J, Trnka A & Capek M. 2024. Chewing lice of Bearded Reedling (Panurus biarmicus) and diversity of louse-host associations of birds in reed beds in Slovakia. Parasite 31, 8.
All Tables
List of hosts and their chewing lice. P/E = prevalence = number of birds parasitized (P)/number of birds examined (E); MI = mean intensity = number of individuals of a particular chewing louse species on infested hosts; MA = mean abundance = number of individuals of a particular chewing louse species on examined birds; Σ = a total number of collected lice; N = nymphs; M = Menoponidae; P = Philopteridae; R = Ricinidae.
All Figures
 |
Figure 1 Menacanthus brelihi Balát, 1981. (A) Female dorso-ventral view; (B) Male dorso-ventral view; (C) Detail of gula; (D) Male genitalia, dorsal view. |
| In the text | |
 |
Figure 2 Menacanthus brelihi Balát, 1981. (A) Holotype female; (B) Allotype male; (C) Type slides; (D) Non-type female. |
| In the text | |
 |
Figure 3 Diagram of the evolution of the Menacanthus lineages. The topology was adapted from the cytochrome oxidase subunit I phylogeny in Supplementary Figure S1. The origin of the samples is in parentheses: AF – Afrotropical realm; EPA – Eastern Palearctic realm; NA – Nearctic realm; NT – Neotropical realm; OR – Oriental realm; WPA – Western Palearctic realm. |
| In the text | |
 |
Figure 4 Penenirmus visendus (Złotorzycka, 1964). (A) Male dorso-ventral view; (B) Female dorso-ventral view. |
| In the text | |
 |
Figure 5 Penenirmus visendus (Złotorzycka, 1964). (A) Male head dorso-ventral view; (B) Dorsal anterior head plate; (C) Female subgenital plate and vulval margin, ventral view; (D), Male genitalia dorsal view. |
| In the text | |
 |
Figure 6 Penenirmus visendus (Złotorzycka, 1964). (A) Holotype female; (B) Holotype slide; (C) Non-type Female; (D) Non-type male. |
| In the text | |
 |
Figure 7 Phylogenetic tree of the Penenirmus species based on partial COI sequences, estimated with Bayesian analysis based on a 379 bp alignment of a COI gene fragment. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The numbers above the branches indicate Bayesian posterior probabilities. Branches with posterior probabilities < 0.5 were collapsed. New sequences are in bold type. The origin of the samples is in parentheses: AF – Afrotropical realm; NA – Nearctic realm; NT – Neotropical realm; OR – Oriental realm; WPA – Western Palearctic realm. |
| In the text | |
 |
Supplementary Figure S1 Phylogenetic tree of the Menacanthus species based on partial COI sequences, estimated with Bayesian analysis based on a 379 bp alignment of a COI gene fragment. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The numbers above the branches indicate Bayesian posterior probabilities. New sequences are in bold type. — Colors: purple – samples from the Western Palearctic realm; dark purple – samples from the Eastern Palearctic realm; red – samples from the Afrotropical realm; green – samples from the Oriental realm; light blue – samples from the Nearctic realm; dark blue – samples from the Neotropical realm. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


