| Issue |
Parasite
Volume 25, 2018
|
|
|---|---|---|
| Article Number | 54 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/parasite/2018058 | |
| Published online | 16 November 2018 | |
Research Article
Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis
Efficacité de la nouvelle stratégie intégrée de contrôle de la transmission de Schistosoma japonicum en Chine : revue systématique et méta-analyse
1
Yuhang Branch, The Second Affiliated Hospital of Zhejiang University, Hangzhou
311100, Zhejiang Province, PR China
2
School of Life Sciences, Fudan University, Shanghai
200433, PR China
3
Department of Clinical Laboratory, The Obstetrics and Gynecology Hospital of Fudan University, Shanghai
200001, PR China
4
Shanghai Skin Disease Hospital, Shanghai
200443, PR China
* Corresponding authors: peachwang814@163.com
Received:
2
August
2018
Accepted:
4
November
2018
Since 2004, the national schistosomiasis control strategy in China has shifted from the morbidity control strategy (conventional strategy) to an integrated strategy (new strategy). We investigated the effectiveness of the new strategy and compared it against the conventional strategy. We retrieved from electronic databases the literature regarding the new strategy published from 2000 to 2017. The effect of the new or conventional strategy on infection by Schistosoma japonicum of humans and snails (Oncomelania hupensis) was evaluated with pooled log relative risk (logRR). A total of only eight eligible publications were included in the final meta-analysis. The results showed that implementation of the new strategy reduced the infection risk by 3–4 times relative to the conventional strategy. More specifically, the conventional strategy caused a reduction in both human (logRR = 0.56, 95% CI: 0.12–0.99) and snail infections (logRR = 0.34, 95% CI: −0.69–1.37), while the new strategy also significantly reduced both human (logRR = 1.89, 95% CI: 1.33–2.46) and snail infections (logRR = 1.61, 95% CI: 1.06–2.15). In contrast to the conventional strategy, the new strategy appeared more effective to control both human (logRR difference = 1.32, 95% CI: 0.78–1.86) and snail infections (logRR difference = 1.53, 95% CI: 0.76–2.31). Our data demonstrate that the new integrated strategy is highly effective to control the transmission of S. japonicum in China, and this strategy is recommended for schistosomiasis elimination in other affected regions across the world, with adaptation to local conditions.
Résumé
Depuis 2004, la stratégie nationale de lutte contre la schistosomiase en Chine est passée d’une stratégie de contrôle de la morbidité (stratégie conventionnelle) à une stratégie intégrée (nouvelle stratégie). Nous avons examiné l’efficacité de la nouvelle stratégie et l’avons comparée à la stratégie conventionnelle. Nous avons extrait des bases de données électroniques la littérature concernant la nouvelle stratégie publiée de 2000 à 2017. L’effet de la stratégie nouvelle ou conventionnelle sur l’infection par Schistosoma japonicum des humains et des mollusques (Oncomelania hupensis) a été évalué avec le risque relatif de log groupé (logRR). Au total, seulement 8 publications éligibles ont été incluses dans la méta-analyse finale. Les résultats ont montré que la mise en œuvre de la nouvelle stratégie réduisait de 3 à 4 fois le risque d’infection par rapport à la stratégie conventionnelle. Plus spécifiquement, la stratégie conventionnelle a entraîné une réduction des infections humaines (logRR = 0.56, IC à 95 % : 0.12–0.99) et des infections des mollusques (logRR = 0.34, IC à 95 % : −0.69–1.37), tandis que la nouvelle stratégie a aussi réduit les infections humaines (logRR = 1.89, IC à 95 % : 1.33 à 2.46) et des mollusques (logRR = 1.61, IC à 95 % : 1.06 à 2.15). Contrairement à la stratégie conventionnelle, la nouvelle stratégie a semblé plus efficace pour contrôler à la fois les infections humaines (différence de logRR = 1.32, IC à 95 % : 0.78–1.86) et les infections des mollusques (différence de logRR = 1.53, IC à 95 % : 0.76–2.31). Nos données démontrent que la nouvelle stratégie intégrée est très efficace pour contrôler la transmission de S. japonicum en Chine et que cette stratégie est recommandée pour l’élimination de la schistosomiase dans d’autres régions touchées du monde, avec une adaptation aux conditions locales.
Key words: Schistosoma japonicum / integrated strategy / morbidity control / effectiveness evaluation / systematic review / meta-analysis
© C. Qian et al., published by EDP Sciences, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Schistosomiasis is a parasitic disease caused by the blood flukes of the genus Schistosoma [8]. It ranks second after malaria among the global human parasitic diseases in terms of socio-economic and public health importance in tropical and subtropical areas [8]. Worldwide, this neglected tropical disease affects more than 207 million people in 78 countries, with 779 million people at risk of infection [37], leading to 0.2 million deaths [29] and 1.75–2.00 million disability adjusted life years (DALYs) each year [18].
Three major Schistosoma species are known to infect humans, including S. haematobium, S. mansoni, and S. japonicum [8]. Schistosomiasis japonica, caused by infection with the parasite S. japonicum, is endemic mainly in China, the Philippines, and parts of Indonesia [8]. Concerted control efforts since the 1950s have dramatically reduced the number of infections as well as the burden of the disease in the endemic areas of China [10, 40, 62]. However, schistosomiasis japonica remains a major public health concern in China, as one of the four priorities for communicable disease control defined by the central government [44]. Currently, the disease remains endemic in the marshland and lake regions of five provinces along the middle and lower reaches of the Yangtze River, and in some mountainous areas in the provinces of Sichuan and Yunnan, and over 0.7 million people living in China are thought to have the disease [63].
The national strategy for schistosomiasis control has shifted three times in China since it was first initiated: transmission control strategy (from mid-1950s to early 1980s), morbidity control strategy (from mid-1980s to 2003), and the new integrated strategy (2004 to present) [53, 54]. The morbidity control strategy, also known as the conventional strategy, focuses on synchronous chemotherapy for humans and bovines [4], and the new strategy developed in 2004 intervenes in the transmission pathway of schistosomiasis japonica, mainly including replacement of bovines with machines, prohibition of grazing cattle in the grasslands, improving sanitation, installation of fecal-matter containers on boats, praziquantel drug therapy, snail control, and health education [42]. This new integrated control strategy has proven to be highly effective to reducing the rate of S. japonicum infection in both humans and the intermediate host snails [24, 26, 39, 43, 46, 65, 66]. However, the effectiveness of this new integrated strategy varies in previous reports in terms of the implementation in different endemic regions and different local circumstances [36]. We therefore present a systematic literature review and meta-analysis to evaluate the effectiveness of the new integrated strategy to control the transmission of S. japonicum in China, and compare results against those of the conventional strategy.
Materials and methods
Search strategy and data source
The studies pertaining to the effectiveness of the new strategy for schistosomiasis control that were published during the period from January 1st, 2000 through December 31th, 2017, were jointly searched in electronic databases, including PubMed, Web of Science, Embase, Proquest, Cochrane Library, China National Knowledge Infrastructure (CNKI), the Wanfang Database and VIP Database. The terms we used included “schistosomiasis”, in combination with “integrated control strategy”, “comprehensive control strategy” or “infectious source control measures”. The title and abstract of each publication screened were read carefully, and the full texts were reviewed.
Study selection
Both inclusion and exclusion criteria were defined for identifying the publications included in our meta-analysis. Inclusion criteria involved: (1) the control measures targeting schistosomiasis japonica; (2) the implementation of the study in China; (3) a detailed description of integrated control interventions with emphasis on control of infectious source of schistosomiasis; (4) inclusion of both study and control areas, and assessment of effectiveness in both groups; (5) a description and evaluation of prevalence of human S. japonicum infection and snail infection as outcomes of the interventions; and (6) available full text for review. The literature articles that met the following criteria were excluded: (1) lack of control areas or lack of effectiveness evaluation in control areas; (2) no description of quantitative outcomes of interventions; (3) the original data regarding the outcomes of interventions were not available; and (4) the full text was unavailable.
Assessment of publication bias
A funnel plot was drawn to evaluate literature quality. We tested funnel plot asymmetry based on the linear regression method [38] using the metabias function in the meta package of R software [34]. We used a cut-off p-value of <0.05 to determine the asymmetry of the funnel plot, and further the presence of publication bias.
Meta-analysis
We carried out a meta-analysis (fixed- or random-effects models) using the RMA function in the metafor package of R software [41]. The effects of the new or conventional strategy in human/snail studies were evaluated with pooled log relative risk (logRR) and the corresponding 95% confidential interval (CI). We then calculated the logRR difference between the strategies and the standard error (SE) as below: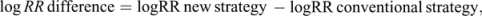
 from which we further compared the two strategies with pooled logRR differences. In all analyses, Cochran’s Q test and I
2 statistics were employed to measure the heterogeneity between studies. A random effects model was employed to estimate overall studies if heterogeneity existed in the data source. Otherwise, a fixed-effect model was reported.
from which we further compared the two strategies with pooled logRR differences. In all analyses, Cochran’s Q test and I
2 statistics were employed to measure the heterogeneity between studies. A random effects model was employed to estimate overall studies if heterogeneity existed in the data source. Otherwise, a fixed-effect model was reported.
All statistical analyses were performed using R software, and a p-value of <0.05 was considered statistically significant.
Results
Literature searched
A total of 1798 publications were identified, of which 147 articles were potentially relevant according to the initial screening. Following the application of the inclusion and exclusion criteria, 139 studies were excluded. Finally, eight papers that examined the effectiveness of the new strategy were included in the meta-analysis (Fig. 1), of which five included two study areas and two control areas. Table 1 describes the general characteristics of the studies enrolled in the analysis [15, 25, 27, 42, 43, 59–61].
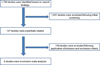 |
Figure 1. Flowchart of study selection. |
Characteristics of the studies included in the meta-analysis
Literature quality
We evaluated the quality of the articles included in this study according to the funnel plot asymmetry using metabias function in the R package meta. Symmetry of the funnel plot was observed, with all p values of >0.05 (Fig. 2). The results indicated no publication bias present in the articles used in the meta-analysis.
 |
Figure 2. Funnel plot shows asymmetry for the studies included in this analysis. (A) the funnel plot of the studies reporting the effectiveness of the conventional strategy on the control of human Schistosoma japonicum infection; (B) the funnel plot of the studies reporting the effectiveness of the conventional strategy on the control of Oncomelania hupensis snail infection; (C) the funnel plot of the studies reporting the effectiveness of the new strategy on the control of human Schistosoma japonicum infection; (D) the funnel plot of the studies reporting the effectiveness of the new strategy on the control of Oncomelania hupensis snail infection; (E) the funnel plot of the studies comparing the effectiveness between the new strategy and the conventional strategy on the control of human Schistosoma japonicum infection; (F) the funnel plot of the studies comparing the effectiveness between the new strategy and the conventional strategy on the control of Oncomelania hupensis snail infection. |
Meta-analysis
A heterogeneity test revealed the presence of heterogeneity among studies that reported the effect of the conventional strategy on the control of human S. japonicum infection (I 2 = 90.34, p < 0.001) and snail infection (I 2 = 83.52, p < 0.001), and the new integrated strategy on the control of human infection (I 2 = 86.39, p < 0.001). No heterogeneity was detected among the studies reporting the alteration of snail infection caused by the new strategy (I 2 = 0.92, p = 0.361). We then estimated pooled logRR and the corresponding 95% CI using random and fixed effects models, respectively.
We found that the implementation of the conventional strategy caused a reduction in both human S. japonicum (logRR = 0.56, 95% CI: 0.12–0.99; Fig. 3A) and snail infections (logRR = 0.34, 95% CI: −0.69–1.37; Fig. 3B), while the new strategy significantly reduced both human S. japonicum (logRR = 1.89, 95% CI: 1.33–2.46; Fig. 4A) and snail infections (logRR = 1.61, 95% CI: 1.06–2.15; Fig. 4B). In other words, the conventional strategy reduced the risk of infection by 1.75-fold (95% CI: 1.13–2.69 fold) in humans and 1.4-fold (95% CI: 0.5–3.94 fold) in snails, while the new strategy reduced 6.62-fold (95% CI: 3.78–11.7 fold) the risk of infection in humans and 5-fold (95% CI: 2.89–8.58 fold) in snails. Further comparison between these two strategies indicated that the new strategy was 3.74-fold (95% CI: 2.18–6.42) (logRR difference = 1.32, 95% CI: 0.78–1.86; Fig. 5A) more effective in human infection control and 4.62-fold (95% CI: 2.14–10.07) (logRR difference = 1.53, 95% CI: 0.76–2.31; Fig. 5B) more effective in snail infection control as compared to the conventional strategy.
 |
Figure 3. Effectiveness of the conventional strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
 |
Figure 4. Effectiveness of the new strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
 |
Figure 5. Comparison of the conventional strategy versus the new strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
Discussion
The description of schistosomiasis in China dates back more than two millennia [64]. Historically, this parasitic disease was called the “god of plagues” by Chairman Mao, the founder of the People’s Republic of China [2, 3]. The disease has caused high social and economic burdens because of its very high rates of morbidity and mortality [56].
The Chinese national schistosomiasis control program was launched in the mid-1950s, and has had three different stages: transmission control strategy, morbidity control strategy, and integrated strategy [53, 54]. In the first stage (from mid-1950s to early 1980s), a transmission control strategy was implemented with emphasis on the control of the intermediate host snails, and mass campaigns were launched to eliminate snail hosts by environmental modification and mollusciciding [13]. During this period, snail habitats were greatly reduced, and the number of schistosomiasis cases decreased [45]. The national schistosomiasis control strategy shifted to morbidity control (from the mid-1980s to 2003) as a response to the advent of the highly effective and low-cost schistosomicide praziquantel [1, 5, 47, 51]. During this stage, five out of the 12 provinces that were endemic for the parasite achieved transmission interruption of schistosomiasis [48]. However, the termination of the World Bank Loan Project for Schistosomiasis Control in 2001 [50] and frequent flooding along the Yangtze River basin [49] resulted in a resurgence of schistosomiasis japonica in China [21, 42, 43]. In order to consolidate the achievements attained and to eliminate schistosomiasis in the country, the Chinese government reinforced the national schistosomiasis control program and prioritized schistosomiasis, together with HIV/AIDS, hepatitis B and tuberculosis in communicable disease control [44]. In addition, a new integrated strategy targeting the transmission pathway of schistosomiasis japonica was proposed to stop environmental contamination with schistosome eggs, which emphasizes replacement of cattle with machines, improvements in sanitation, and fencing of water buffaloes, along with health education, praziquantel-based drug therapy and snail control [42].
The new integrated strategy was designed to reduce the role of cattle and humans as sources of S. japonicum infection [42]. It has been highly effective in controlling the transmission of S. japonicum in the endemic foci of China [6, 11, 14, 20, 58, 65]. Since the new strategy was implemented in various endemic regions and different combinations of interventions were adopted, the effectiveness of the strategy in reducing infection by S. japonicum in humans and the intermediate host snails has been found to vary in previous studies. However, there has been no systematic evaluation of this new strategy to control the transmission of S. japonicum in China until now. We therefore carried out a systematic literature review and meta-analysis with the aim of performing a pooled analysis of the effectiveness of the new strategy, and comparing the effectiveness of the new strategy with the conventional strategy in reducing S. japonicum infection in both humans and snails.
Our meta-analysis showed that the implementation of the conventional strategy caused a reduction in both human S. japonicum infection (logRR = 0.56, 95% CI: 0.12–0.99) and snail infection (logRR = 0.34, 95% CI: –0.69–1.37), suggesting that the praziquantel-based morbidity control strategy is effective in reducing S. japonicum infection in humans and snails, while the new strategy remarkably reduced both human S. japonicum (logRR = 1.89, 95% CI: 1.33–2.46) and snail infections (logRR = 1.61, 95% CI: 1.06–2.15), indicating that the integrated strategy with emphasis on controlling the source of S. japonicum infection is effective in controlling the transmission of S. japonicum. However, the new strategy appeared more effective in controlling both human S. japonicum (logRR difference = 1.32, 95% CI: 0.78–1.86) and snail infections (logRR difference = 1.53, 95% CI: 0.76–2.31) than the conventional strategy.
The morbidity control strategy mainly involves praziquantel-based drug therapy, snail control, and health education interventions [28]. Nevertheless, praziquantel is ineffective in preventing S. japonicum infection and re-infection [23], and it is unlikely to eliminate snails completely in the endemic foci [62]. China’s experiences and lessons from the past three decades of schistosomiasis control have shown that the morbidity control strategy is insufficient to eliminate schistosomiasis in the country [62, 67]. In the Philippines, mass drug administration with praziquantel on its own has proven to be ineffective to control the prevalence of schistosomiasis, the intensity of S. japonicum infection, or the morbidity of the disease [17, 31, 32]. Moreover, praziquantel-based deworming alone has been proved ineffective to eliminate schistosomiasis from the African mainland [7, 9, 16, 35]. These findings demonstrate that the sustainable control and elimination of schistosomiasis requires an integrated, multidisciplinary and multi-component strategy [30].
The integrated strategy relies on the fact that cattle have been considered as the major infectious source for the transmission of schistosomiasis in the marshland and lake regions of China [12, 22]. It is therefore assumed that the successful intervention packages piloted in the marshland and lake regions are not fully suitable for the hilly and mountainous environments in the Sichuan and Yunnan provinces of China [36]. Although field studies have shown that this new integrated strategy remains effective to control S. japonicum infection in humans and snails in hilly and mountainous endemic foci [26, 27, 57], regionally flexible integrated, intersectoral, and setting-specific control strategies driven by local circumstances and data are needed [36].
The present study has some limitations. First, only eight eligible studies were enrolled in the meta-analysis. A total of 147 potentially relevant literatures were initially identified; however, 139 studies were excluded due to unavailability of original data regarding S. japonicum infection in humans and snails in the articles. In addition, most of the studies were published in national journals. Therefore, more randomized controlled trials with a rigorous design to evaluate the effect of the integrated control strategy for schistosomiasis japonica seem justified, and the research outcomes are encouraged to be transferred around the world. Second, no stratified analysis was performed. Since there were only eight studies included in the meta-analysis, we evaluated the effectiveness of the new integrated strategy implemented in endemic foci with various endemic types, and did not assess the endemic type-specific effectiveness. Further systematic evaluations recruiting more trials to evaluate the effectiveness of the new integrated strategy for controlling the transmission of S. japonicum in the marshland and lake regions, the mountainous regions and plain regions, respectively, seem justified.
In summary, the results of the present study demonstrate that the new integrated strategy with emphasis on the control of the infectious source is highly effective to control the transmission of S. japonicum in China. The elimination of schistosomiasis japonica in the country requires continually effective and extensive implementation of an integrated, intersectoral, and setting-specific control strategy. Currently, China is transferring its expertise in schistosomiasis control to Africa, and the Philippines may also learn much from China’s experiences and lessons [52, 55]. Experiences and lessons from China are important for shaping the schistosomiasis elimination agenda [19]. However, there is still a need to devise an optimal control strategy with adaptation to local circumstances to facilitate the progress towards the elimination of schistosomiasis in Africa and the Philippines [33].
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81601831 and 81602778), Shanghai Municipal Commission of Health and Family Planning (Grant No. 2015-4Y0055), Hangzhou Municipal Scientific Research Project for Medical Sciences (Grant No. 20150633B66), Yuhang District Key Scientific Research Project for Medical Sciences (Grant No. 2015008) and Yuhang District Key Department for Medical Sciences (Grant No. 2017005).
References
- Bergquist R, Utzinger J, Keiser J. 2017. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infectious Diseases of Poverty, 6, 74. [CrossRef] [PubMed] [Google Scholar]
- Berry-Cabán CS. 2007. Return of the god of plague: schistosomiasis in China. Journal of Rural and Tropical Public Health, 6, 45–53. [Google Scholar]
- Bundy DA, Gottlieb M. 1999. Parasitic infection in China: farewell to the god of plagues. Parasitology Today, 15, 170–172. [CrossRef] [Google Scholar]
- Chen MG. 2005. Use of praziquantel for clinical treatment and morbidity control of schistosomiasis japonica in China: a review of 30years’ experience. Acta Tropica, 96, 168–176. [CrossRef] [PubMed] [Google Scholar]
- Chen MG. 2014. Assessment of morbidity due to Schistosoma japonicum infection in China. Infectious Diseases of Poverty, 3, 6. [CrossRef] [PubMed] [Google Scholar]
- Chen YY, Liu JB, Huang XB, Cai SX, Su ZM, Zhong R, Zou L, Miao XP. 2014. New integrated strategy emphasizing infection source control to curb Schistosomiasis japonica in a marshland area of Hubei Province, China: findings from an eight-year longitudinal survey. PLoS One, 9, e89779. [CrossRef] [PubMed] [Google Scholar]
- Colley DG. 2014. Morbidity control of schistosomiasis by mass drug administration: how can we do it best and what will it take to move on to elimination? Tropical Medicine and Health, 42, 25–32. [CrossRef] [PubMed] [Google Scholar]
- Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet, 383, 2253–2264. [CrossRef] [PubMed] [Google Scholar]
- Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, Coles G, TchuemTchuenté LA, Mbaye A, Engels D. 2009. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology, 136, 1825–1835. [CrossRef] [PubMed] [Google Scholar]
- Engels D, Wang LY, Palmer KL. 2005. Control of schistosomiasis in China. Acta Tropica, 96, 67–68. [CrossRef] [PubMed] [Google Scholar]
- Gray DJ, Li YS, Williams GM, Zhao ZY, Harn DA, Li SM, Ren MY, Feng Z, Guo FY, Guo JG, Zhou J, Dong YL, Li Y, Ross AG, McManus DP. 2014. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: design and baseline results of a 4-year cluster-randomised intervention trial. International Journal for Parasitology, 44, 659–668. [CrossRef] [PubMed] [Google Scholar]
- Gray DJ, Williams GM, Li Y, McManus DP. 2008. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS One, 3, e4058. [CrossRef] [PubMed] [Google Scholar]
- Hipgrave D. 2011. Communicable disease control in China: from Mao to now. Journal of Global Health, 1, 224–238. [PubMed] [Google Scholar]
- Hong QB, Yang K, Huang YX, Sun LP, Yang GJ, Gao Y, Gao Y, Zhang LH, Zhou M, Steinmann P, Liang YS. 2011. Effectiveness of a comprehensive schistosomiasis japonica control program in Jiangsu province, China, from 2005 to 2008. Acta Tropica, 120, S151–S157. [CrossRef] [PubMed] [Google Scholar]
- Hong XC, Xu XJ, Chen X, Li YS, Yu CH, Yuan Y, Chen YY, Li RD, Qiu J, Liu ZC, Yi P, Ren GH, He HB. 2013. Assessing the effect of an integrated control strategy for schistosomiasis japonica emphasizing bovines in a marshland area of Hubei Province, China: a cluster randomized trial. PLoS Neglected Tropical Diseases, 7, e2122. [CrossRef] [PubMed] [Google Scholar]
- Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Neglected Tropical Diseases, 3, e412. [CrossRef] [PubMed] [Google Scholar]
- Inobaya MT, Olveda RM, Tallo V, McManus DP, Williams GM, Harn DA, Li Y, Chau TN, Olveda DU, Ross AG. 2015. Schistosomiasis mass drug administration in the Philippines: lessons learnt and the global implications. Microbes and Infection, 17, 6–15. [CrossRef] [PubMed] [Google Scholar]
- King CH. 2010. Parasites and poverty: the case of schistosomiasi. Acta Tropica, 113, 95–104. [CrossRef] [PubMed] [Google Scholar]
- King CH. 2017. The evolving schistosomiasis agenda 2007–2017 – Why we are moving beyond morbidity control toward elimination of transmission. PLoS Neglected Tropical Diseases, 11, e0005517. [CrossRef] [PubMed] [Google Scholar]
- Li SZ, Qian YJ, Yang K, Wang Q, Zhang HM, Liu J, Chen MH, Huang XB, Xu YL, Bergquist R, Zhou XN. 2012. Successful outcome of an integrated strategy for the reduction of schistosomiasis transmission in an endemically complex area. Geospatial Health, 6, 215–220. [CrossRef] [PubMed] [Google Scholar]
- Liang S, Yang C, Zhong B, Qiu D. 2006. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bulletin of the World Health Organization, 84, 139–144. [CrossRef] [PubMed] [Google Scholar]
- Liu J, Zhu C, Shi Y, Li H, Wang L, Qin S, Kang S, Huang Y, Jin Y, Lin J. 2012. Surveillance of Schistosoma japonicum infection in domestic ruminants in the Dongting Lake region, Hunan province, China. PLoS One, 7, e31876. [CrossRef] [PubMed] [Google Scholar]
- Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS. 2011. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. Parasites and Vectors, 4, 201. [CrossRef] [Google Scholar]
- Liu R, Dong HF, Jiang MS. 2013. The new national integrated strategy emphasizing infection sources control for schistosomiasis control in China has made remarkable achievements. Parasitology Research, 112, 1483–1491. [CrossRef] [PubMed] [Google Scholar]
- Liu W, Cao CL, Chen Z, Li SZ, Tang L, Xiao Y, Zhang HM, Yang ZQ, Wang Y, Su SY, Wang LY, Wang Q, Xu JF, Bao ZP, Huang XB, Zhou XN. 2013. Evaluation of the comprehensive schistosomiasis control measures with emphasis on infection source of replacing cattle with machine. Chinese Journal of Parasitology and Parasitic Diseases, 31, 296–301. [Google Scholar]
- Liu Y, Zhong B, Wu ZS, Liang S, Qiu DC, Ma X. 2017. Interruption of schistosomiasis transmission in mountainous and hilly regions with an integrated strategy: a longitudinal case study in Sichuan, China. Infectious Diseases of Poverty, 6, 79. [CrossRef] [PubMed] [Google Scholar]
- Luo TP, Zhou XN, Qiu ZL. 2009. Cost-effectiveness and cost-benefit of integrated schistosomiasis control strategy with emphasis on infectious source control in mountainous areas of Yunnan Province. Chinese Journal of Schistosomiasis Control, 21, 93–97. [Google Scholar]
- Qing-Wu J, Li-Ying W, Jia-Gang G, Ming-Gang C, Xiao-Nong Z, Engels D. 2002. Morbidity control of schistosomiasis in China. Acta Tropica, 82, 115–125. [CrossRef] [PubMed] [Google Scholar]
- Rollinson D, Knopp S, Levitz S, Stothard JR, TchuemTchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. 2013. Time to set the agenda for schistosomiasis elimination. Acta Tropica, 128, 423–440. [CrossRef] [PubMed] [Google Scholar]
- Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. 2017. A new global strategy for the elimination of schistosomiasis. International Journal of Infectious Diseases, 54, 130–137. [CrossRef] [Google Scholar]
- Ross AG, Olveda RM, Acosta L, Harn DA, Chy D, Li Y, Gray DJ, Gordon CA, McManus DP, Williams GM. 2013. Road to the elimination of schistosomiasis from Asia: the journey is far from over. Microbes and Infection, 15, 858–865. [CrossRef] [PubMed] [Google Scholar]
- Ross AG, Olveda RM, Chy D, Olveda DU, Li Y, Harn DA, Gray DJ, McManus DP, Tallo V, Chau TN, Williams GM. 2015. Can mass drug administration lead to the sustainable control of schistosomiasis? Journal of Infectious Diseases, 211, 283–289. [CrossRef] [Google Scholar]
- Savioli L, Albonico M, Colley DG, Correa-Oliveira R, Fenwick A, Green W, Kabatereine N, Kabore A, Katz N, Klohe K, LoVerde PT, Rollinson D, Stothard JR, Tchuem Tchuenté LA, Waltz J, Zhou XN. 2017. Building a global schistosomiasis alliance: an opportunity to join forces to fight inequality and rural poverty. Infectious Diseases of Poverty, 6, 65. [CrossRef] [PubMed] [Google Scholar]
- Schwarzer G. 2013. meta: Meta-Analysis with R.R package version 3.0-1. http://CRAN.R-project.org/package=meta. [Google Scholar]
- Sesay S, Paye J, Bah MS, McCarthy FM, Conteh A, Sonnie M, Hodges MH, Zhang Y. 2014. Schistosoma mansoni infection after three years of mass drug administration in Sierra Leone. Parasites and Vectors, 7, 14. [CrossRef] [Google Scholar]
- Seto EY, Remais JV, Carlton EJ, Wang S, Liang S, Brindley PJ, Qiu D, Spear RC, Wang LD, Wang TP, Chen HG, Dong XQ, Wang LY, Hao Y, Bergquist R, Zhou XN. 2011. Toward sustainable and comprehensive control of schistosomiasis in China: lessons from Sichuan. PLoS Neglected Tropical Diseases, 5, e1372. [CrossRef] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infectious Diseases, 6, 411–425. [CrossRef] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. 2011. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. British Medical Journal, 343, d4002. [CrossRef] [PubMed] [Google Scholar]
- Sun LP, Wang W, Liang YS, Tian ZX, Hong QB, Yang K, Yang GJ, Dai JR, Gao Y. 2011. Effect of an integrated control strategy for schistosomiasis japonica in the lower reaches of the Yangtze River, China: an evaluation from 2005 to 2008. Parasites and Vectors, 4, 243. [CrossRef] [Google Scholar]
- Utzinger J, Zhou XN, Chen MG, Bergquist R. 2005. Conquering schistosomiasis in China: the long march. Acta Tropica, 96, 69–96. [CrossRef] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. 2009. A strategy to control transmission of Schistosoma japonicum in China. New England Journal of Medicine, 360, 121–128. [CrossRef] [PubMed] [Google Scholar]
- Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP, Wu ZD, Wang LY, Hao Y, Bergquist R, Utzinger J, Zhou XN. 2009. China’s new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Tropical Medicine and International Health, 14, 1475–1483. [CrossRef] [Google Scholar]
- Wang LD, Utzinger J, Zhou XN. 2008. Schistosomiasis control: experiences and lessons from China. Lancet, 372, 1793–1795. [CrossRef] [PubMed] [Google Scholar]
- Wang W, Dai JR, Liang YS. 2014. Apropos: factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasites and Vectors, 7, 408. [CrossRef] [Google Scholar]
- Wang X, Wang W, Wang P. 2017. Long-term effectiveness of the integrated schistosomiasis control strategy with emphasis on infectious source control in China: a 10-year evaluation from 2005 to 2014. Parasitology Research, 116, 521–528. [CrossRef] [PubMed] [Google Scholar]
- Wu W, Wang W, Huang YX. 2011. New insight into praziquantel against various developmental stages of schistosomes. Parasitology Research, 109, 1501–1507. [CrossRef] [PubMed] [Google Scholar]
- Wu XH, Chen MG, Zheng J. 2005. Surveillance of schistosomiasis in five provinces of China which have reached the national criteria for elimination of the disease. Acta Tropica, 96, 276–281. [CrossRef] [PubMed] [Google Scholar]
- Wu XH, Zhang SQ, Xu XJ, Huang YX, Steinmann P, Utzinger J, Wang TP, Xu J, Zheng J, Zhou XN. 2008. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitology International, 57, 271–276. [CrossRef] [PubMed] [Google Scholar]
- Xianyi C, Liying W, Jiming C, Xiaonong Z, Jiang Z, Jiagang G, Xiaohua W, Engels D, Minggang C. 2005. Schistosomiasis control in China: the impact of a 10-year World Bank Loan Project (1992–2001). Bulletin of the World Health Organization, 83, 43–48. [PubMed] [Google Scholar]
- Xiao SH, Keiser J, Chen MG, Tanner M, Utzinger J. 2010. Research and development of antischistosomal drugs in the People’s Republic of China a 60-year review. Advances in Parasitology, 73, 231–295. [CrossRef] [PubMed] [Google Scholar]
- Xu J, Bergquist R, Qian YJ, Wang Q, Yu Q, Peeling R, Croft S, Guo JG, Zhou XN. 2016. China-Africa and China-Asia Collaboration on schistosomiasis control: a SWOT analysis. Advances in Parasitology, 92, 435–466. [CrossRef] [PubMed] [Google Scholar]
- Xu J, Steinman P, Maybe D, Zhou XN, Lv S, Li SZ, Peeling R. 2016. Evolution of the National Schistosomiasis Control Programmes in the People’s Republic of China. Advances in Parasitology, 92, 1–38. [CrossRef] [PubMed] [Google Scholar]
- Xu J, Xu JF, Li SZ, Zhang LJ, Wang Q, Zhu HH, Zhou XN. 2015. Integrated control programmes for schistosomiasis and other helminth infections in P.R. China. Acta Tropica, 141, 332–341. [CrossRef] [PubMed] [Google Scholar]
- Xu J, Yu Q, Tchuenté LA, Bergquist R, Sacko M, Utzinger J, Lin DD, Yang K, Zhang LJ, Wang Q, Li SZ, Guo JG, Zhou XN. 2016. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infectious Diseases, 16, 376–383. [CrossRef] [Google Scholar]
- Yang GJ, Liu L, Zhu HR, Griffiths SM, Tanner M, Bergquist R, Utzinger J, Zhou XN. 2014. China’s sustained drive to eliminate neglected tropical diseases. Lancet Infectious Diseases, 14, 881–892. [CrossRef] [Google Scholar]
- Yang K, Li Hong J, Yang WC, Shi XW, Qi YL. 2009. Effect of comprehensive schistosomiasis control measures with emphasis on infectious source control in dam areas of mountainous region, Yunnan Province. Chinese Journal of Schistosomiasis Control, 21, 272–275. [Google Scholar]
- Yang Y, Zhou YB, Song XX, Li SZ, Zhong B, Wang TP, Bergquist R, Zhou XN, Jiang QW. 2016. Integrated control strategy of schistosomiasis in the People’s Republic of China: projects involving agriculture, water conservancy, forestry, sanitation and environmental modification. Advances in Parasitology, 92, 237–268. [CrossRef] [PubMed] [Google Scholar]
- Yu LQ, Qiu XY, Hu Y, Wang YL. 2010. Effect of replacing bovine with tractors for farming on schistosomiasis control in Xuancheng City. Chinese Journal of Schistosomiasis Control, 22, I–II [Google Scholar]
- Yu Q, Zhao GM, Hong XL, Lutz EA, Guo JG. 2013. Impact and cost-effectiveness of a comprehensive schistosomiasis japonica control program in the Poyang Lake region of China. International Journal of Environmental Research and Public Health, 10, 6409–6421. [CrossRef] [PubMed] [Google Scholar]
- Zhang SQ, Wang TP, Tao CG, Chen GX, Chen JS, Xu H, Yin NW, Wang H, Ge JH. 2005. Observation on comprehensive measures of safe treatment of night-soil and water supply, replacement of bovine with machine for schistosomiasis control. Chinese Journal of Schistosomiasis Control, 17, 437–442. [Google Scholar]
- Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, Olveda R. 2010. Schistosomiasis japonica control and research needs. Advances in Parasitology, 72, 145–148. [CrossRef] [PubMed] [Google Scholar]
- Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y. 2007. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerging Infectious Diseases, 13, 1470–1476. [CrossRef] [PubMed] [Google Scholar]
- Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. 2005. The public health significance and control of schistosomiasis in China – then and now. Acta Tropica, 96, 97–105. [CrossRef] [PubMed] [Google Scholar]
- Zhou YB, Liang S, Chen GX, Rea C, He ZG, Zhang ZJ, Wei JG, Zhao GM, Jiang QW. 2011. An integrated strategy for transmission control of Schistosoma japonicum in a marshland area of China: findings from a five-year longitudinal survey and mathematical modeling. American Journal of Tropical Medicine and Hygiene, 85, 83–88. [CrossRef] [Google Scholar]
- Zhou YB, Liang S, Chen GX, Rea C, Han SM, He ZG, Li YP, Wei JG, Zhao GM, Jiang QW. 2013. Spatial-temporal variations of Schistosoma japonicum distribution after an integrated national control strategy: a cohort in a marshland area of China. BMC Public Health, 13, 297. [CrossRef] [PubMed] [Google Scholar]
- Zou L, Ruan S. 2015. Schistosomiasis transmission and control in China. Acta Tropica, 143, 51–57. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Qian C, Zhang Y, Zhang X, Yuan C, Gao Z, Yuan H & Zhong J. 2018. Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis. Parasite 25, 54.
All Tables
All Figures
 |
Figure 1. Flowchart of study selection. |
| In the text | |
 |
Figure 2. Funnel plot shows asymmetry for the studies included in this analysis. (A) the funnel plot of the studies reporting the effectiveness of the conventional strategy on the control of human Schistosoma japonicum infection; (B) the funnel plot of the studies reporting the effectiveness of the conventional strategy on the control of Oncomelania hupensis snail infection; (C) the funnel plot of the studies reporting the effectiveness of the new strategy on the control of human Schistosoma japonicum infection; (D) the funnel plot of the studies reporting the effectiveness of the new strategy on the control of Oncomelania hupensis snail infection; (E) the funnel plot of the studies comparing the effectiveness between the new strategy and the conventional strategy on the control of human Schistosoma japonicum infection; (F) the funnel plot of the studies comparing the effectiveness between the new strategy and the conventional strategy on the control of Oncomelania hupensis snail infection. |
| In the text | |
 |
Figure 3. Effectiveness of the conventional strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
| In the text | |
 |
Figure 4. Effectiveness of the new strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
| In the text | |
 |
Figure 5. Comparison of the conventional strategy versus the new strategy on the control of human Schistosoma japonicum infection (A) and Oncomelania hupensis snail infection (B). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


