| Issue |
Parasite
Volume 20, 2013
|
|
|---|---|---|
| Article Number | 40 | |
| Number of page(s) | 5 | |
| DOI | https://doi.org/10.1051/parasite/2013036 | |
| Published online | 24 October 2013 | |
Research Article
Neospora caninum antibodies in dairy cows and domestic dogs from Vojvodina, Serbia
Anticorps contre Neospora caninum chez les vaches laitières et les chiens de Voïvodine, Serbie
1
Faculty of Agriculture, University of Novi Sad, Trg Dositeja Obradovića 8, 21000 Novi Sad, Serbia
2
PVP MSV Medicus D.O.O., Milice Stojadinović Srpkinje 1, 21209 Bukovac, Serbia
3
Praxis Veterinaria D.O.O., 2. Oktobra 40, 26300 Vršac, Serbia
* Corresponding author: pavicic.ljiljana@gmail.com
Received:
28
June
2013
Accepted:
5
October
2013
Neospora caninum, the causative agent of neosporosis, is a protozoan parasite responsible for high rate of abortion in cattle worldwide. In dogs, consequences of infection vary from severe neuromuscular disorders to asymptomatic infection and shedding of environmentally resistant oocysts. In this study, we determined the occurrence of N. caninum antibodies in dairy cattle and dogs in Vojvodina (Northern Province of Serbia) and possible risk factors. N. caninum antibodies were found in 15.4% (55/356, CI 95%:12.0–19.6) of cows and 17.2% (17/99, CI 95%: 10.8–26.2) of dogs. Cows from smallholdings showed significantly greater odds (OR = 5.28, CI 95%: 2.0–13.6, p = 0.0006) of being seropositive in comparison to the farm cows. Epidemiological importance of results is discussed.
Résumé
Neospora caninum, l’agent qui cause la néosporose, est un parasite protozoaire responsable d’un taux élevé d’avortements dans le bétail à travers le monde. Chez les chiens, les conséquences de l’infection varient entre un trouble neuromusculaire sévère et une excrétion asymptomatique d’oocystes résistants dans l’environnement. L’objectif de cette étude était d’examiner le statut sérologique des vaches laitières et des chiens en Voïvodine (province du nord de la Serbie) et les facteurs de risque possibles. Des anticorps contre N. caninum ont été détectés dans 15,4 % (55/356, CI 95 %:12,0–19,6) des vaches et dans 17,2 % (17/99, CI 95 %:10,8–26,2) des chiens. Les vaches des petits propriétaires ont manifesté une probabilité significativement plus importante (OR = 5,28, CI 95 %:2,0–13,6, p = 0,0006) d’être séropositives que les vaches de fermes. L’importance épidémiologique de ces résultats est discutée.
Key words: Anti-Neospora caninum antibodies / Dairy cows / Dogs / Risk factors / Vojvodina
© L. Kuruca et al., published by EDP Sciences, 2013
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The protozoan parasite Neospora caninum causes considerable economic losses to cattle industry worldwide [1].
One of the definitive hosts of N. caninum, which is responsible for shedding of environmentally resistant oocysts, is the domestic dog [6]. In dogs, neosporosis can cause severe clinical manifestations, including myositis-polyradiculoneuritis, encephalomyelitis and dermatitis [7, 12].
Worldwide reports of clinical and subclinical infections in all hosts were summarized by Dubey et al. [1] and Dubey and Schares [2], but there was no mention of neosporosis in Serbia. Recently, occurrence of N. caninum specific antibodies in both cattle and dogs has been confirmed in Serbia as well [3, 9, 13, 17] and published in local journals. The aim of this study was to investigate current serological status of dairy cattle and dogs in Vojvodina (Northern Province of Serbia), with regard to the possible risk factors.
Material and methods
Studied area
Vojvodina is a northern (45°15′ N 19°50′ E) province of the Republic of Serbia which occupies 21,506 km2 of the state territory [5]. The major part of the province’s territory consists of fertile plains with the Danube, Tisa and Sava rivers dividing it in to three regions: Bačka, Banat and Srem. The climate of Vojvodina is moderately continental, characterised by hot, dry summers, cold winters and relatively low rainfall.
Animals and sample collection
Blood samples were collected from 356 dairy cows from both commercial farms (109 cows) and smallholdings (247 cows) in Srem, Banat and Bačka region (Figure 1), during the 2009–2013 period. Samples from 271 cows were obtained on the farm by jugular venipuncture and 85 samples were collected at the abattoir. Among these were 74 samples from cows with a history of various reproductive disorders and 197 samples from reproductively healthy cows. Medical history could not be obtained for 85 abattoir samples. Prior to sampling, minimum recommended size of the sample was calculated, using Win Episcope 2.0 software [16]. Announcement of the Statistical Office of the Republic of Serbia on the number of cattle [14] and expected prevalence of 17.3% [13] that would ensure a 95% confidence interval and produce an error of 5% were used as input data for this calculation. Minimum recommended size of 220 animals was obtained. Therefore, a sample of 356 animals would not only provide an unbiased estimation of the prevalence of N. caninum-specific antibodies in the dairy cows of Vojvodina but would also reduce the error to 3.92%.
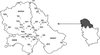 |
Figure 1. Dots on the map of Vojvodina represent localities where blood samples were taken from cows or, in the case of blood collected at the abattoir, places of cows’ origin. Black dots refer to localities where seropositive cows were found, while white dots stand for localities where there were no positive samples. Figures represent the number of sampled cows from specific locality and number of positive ones (between parentheses). |
Blood samples were obtained from 99 dogs during the 2008–2013 period, from various sources on several locations in Srem, Banat and Bačka (Figure 2). None of the sampled dogs, including six farm dogs, belonged to the same farms as cows from this study. All canine blood samples were collected from cephalic vein. The dogs were clinically examined prior to sampling and for each dog a record sheet, which included information on breed, gender, age, previous health problem and diet, was filled. For stray dogs, only gender and age (estimated by competent veterinarian) could be obtained.
 |
Figure 2. Geographical distribution of localities where blood samples were collected from hunting, farm and stray dogs. Numbers outside parentheses represent the dogs from the specific category that was tested on the given locality. Numbers between parentheses refer to the positive ones. |
Sample preparation procedure was identical for both cows and dogs: after being left to clot, blood was centrifuged (3000 rpm for 10 min) and separated sera were stored at −20 °C until examination.
Serological testing
Two types of serological tests were used to detect N. caninum antibodies in cow sera. Initially 100 sera were assayed using the commercial competitive ELISA test kit (cELISA, VMRD Inc., Pullman, USA). The remainder 256 cow and all dog sera were examined with an indirect fluorescent antibody test (IFAT) using reagents marketed by VMRD. Both tests were performed according to the manufacturer’s instructions. Cow sera, examined with ELISA, that presented inhibition percentages equal to or higher than 30% were considered positive. For IFAT, a recommended cut-off of 1:200 for cows and 1:50 for dogs was used. Dog sera that exhibited positive reaction at 1:50 were serially diluted until negativity was reached.
Statistical analysis
Seroprevalences and their confidence intervals, for both cows and dogs, were calculated using Quantitative Parasitology 3.0. [11]. For the statistical analysis of the possible effects of different factors (origin, farm type and history of reproductive disorders in cows and utilisation, breed, gender, age, origin and feeding habits in dogs) on the occurrence of anti-N. caninum antibodies the chi-squared test was used at a significance level of 95% (p ≤ 0.05). Where appropriate chi-squared test was replaced by Fisher’s exact test or unconditional exact test. All these tests were computed using the above mentioned statistical software. Odds ratio (OR) was calculated using Win Episcope version 2.0 software [16]. Positive OR (OR > 1), with 95% confidence interval (CI) that does not overlap with the null value of 1 and with p ≤ 0.05, was considered statistically significant [15]. Confidence interval and p-value for OR were calculated using online MedCalc software version 12.6 [8].
Results
Antibodies to N. caninum were found in 15.4% (55/356, CI 95%:12.0–19.6) of cow sera. Seven positive sera were detected by ELISA and the remaining 48 by IFAT (Table 1). Among the risk factors evaluated, only cows originating from smallholdings had significantly greater odds (OR = 5.28, CI 95%: 2.0–13.6, p = 0.0006) of being seropositive in comparison to the group of farm cows.
Prevalence of seropositive cows according to the geographical origin, farm type and health status.
N. caninum antibodies were found in 17.2% (17/99, CI 95%: 10.8–26.2) of dogs, with titres of 50 in 15 dogs, 100 in two and 200 in one dog. Out of 17 seropositive animals, 14 (14/71, 19.7%, CI 95%: 11.2–30.9) came from the group of hunting dogs, one (1/22, 4.5%, CI 95%: 0.2–22.2) was a stray dog and two (2/6, 33.3%, CI 95%: 6.3–72.9) belonged to a small group of farm dogs. Of all the risk factors evaluated statistical difference (p = 0.02) was observed in the occurrence of N. caninum-specific antibodies in dogs from Bačka versus dogs from Banat (Table 2).
Prevalence of anti-Neospora caninum antibodies in hunting, stray and farm dogs according to the breed, gender, age and region of origin.
In 42 hunting dogs with known feeding habits 11 (26.2%, CI 95%: 14.9–41.6) were seropositive. No statistical differences (p = 0.4) were found between the dogs fed commercial food (2/12, 16.7%, CI 95%: 2.1–48.4), homemade food (8/22, 36.4%, CI 95%: 17.2–59.3) and the mixture of two diets (1/8, 12.5%, CI 95%: 0.6–50.0).
Seropositive purebred dogs were present as follows: Dachshund (n = 1), English Springer Spaniel (n = 1), English Pointer (n = 1), German Shorthaired Pointer (n = 4), German Hunting Terrier (n = 1), Weimaraner (n = 1), Setter (n = 1), German Wirehaired Pointer (n = 2), Labrador Retriever (n = 1), Small Munsterlander Pointer (n = 1) and German Shepherd (n = 1).
Most (92 of 99) dogs were clinically normal and seven dogs had clinical signs which could not be related to neosporosis.
Discussion
The results of this study corroborate previous findings [3, 9, 13, 17] of N. caninum antibodies in both cattle and dogs from the territory of Vojvodina.
The prevalence of antibodies in dairy cattle from our study (15.4%) was higher than the one found by Gavrilović et al. [3]. Similar to our study, their sample consisted of both aborting and randomly sampled cows, from both commercial and smallholding farms. Nevertheless, they found only 4.6% (23/500) of cows to be seropositive, which could be partly due to the small proportion of aborting cows in the total sample and the fact that they restricted their research to the south district of Banat. Different commercial tests were utilized in these studies (Gavrilović et al. used ELISA manufactured by IDEXX and Svanova, while we used IFAT and ELISA manufactured by VMRD) which may have also influenced the results. Vidić et al. [17] and Savović et al. [13] reported occurrence of N. caninum antibodies in 3.7% (5/132) and 17.3% (9/52) of dairy cows, respectively. Although small size of the study samples and the fact they consisted entirely of cows with reproductive disorders make these prevalences difficult to compare with, we referred to them in the interest of data transparency.
With regard to the risk factors, significant difference was observed between seroprevalences in cattle from commercial and from small farms in our study. Probable reason for these observations is the fact that biosecurity measures on commercial farms are generally superior to those on small family farms which are more likely to have dogs, the potential source of infection. Furthermore, cattle from smallholdings are usually associated with extensive production and often allowed to graze, which is, according to some authors [4, 10], one of the risk factors associated with seropositivity in cattle. Gavrilović et al. [3] also observed that all cows from seropositive commercial farms in their study had access to the outdoor paddocks which resulted in higher herd prevalence in commercial farms (50.0%), in comparison to smallholdings (22.64%). On individual level, however, they found no significant difference between commercial (4.67%) and small (4.50%) farms.
Prevalence of N. caninum antibodies in dogs varies based on many factors, including age, origin and diet [1]. Pavičić et al. [9] were first to detect N. caninum antibodies in four out of 31 (12.9%) dogs from Vojvodina. Examined dogs were mostly hounds, originating from Srem and Banat region. In the present study, we found antibodies in 17 out of 99 dogs (17.2%). Samples were collected from all three regions of Vojvodina and higher prevalence was observed in dogs from Bačka (26.1%) in comparison to dogs from Banat (7%). Other factors, such as breed, age, diet or utilisation did not appear significant. However, since the sample size was relatively small and samples originated from various sources and were collected over several years, no definitive conclusion can be made. Nevertheless, we recorded findings for the benefit of future studies.
Presently, there are no reports of confirmed clinical neosporosis in any host in Serbia, including cattle and dogs.
In conclusion, our study confirmed the presence of N. caninum antibodies in general population of dairy cows in Vojvodina, with a higher prevalence detected in animals originating from smallholding farms. Specific antibodies against N. caninum were also found in dogs from the same province, with slightly higher prevalence observed in dogs from Bačka.
Acknowledgments
To the Ministry of Education, Science and Technological Development of the Republic of Serbia which supported this study with Grants No. TR31034 (PhD grant for Ljiljana Kuruca) and No. TR31084.
References
- Dubey JP, Schares G, Ortega-Mora LM. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clinical Microbiology Reviews, 20, 323–367. [CrossRef] [PubMed] [Google Scholar]
- Dubey JP, Schares G. 2011. Neosporosis in animals – the last five years. Veterinary Parasitology, 180, 90–108. [CrossRef] [PubMed] [Google Scholar]
- Gavrilović P, Živulj A, Todorović I, Jovanović M, Parunović J. 2013. Investigation of importance of Neospora caninum in aetiology of abortion in dairy cows in Serbia. Revue de Médecine Vétérinaire, 164, 100–104. [Google Scholar]
- Guimarães JS Jr, Souza SLP, Bergamaschi DP, Gennari SM. 2004. Prevalence of Neospora caninum antibodies and factors associated with their presence in dairy cattle of the north of Paraná state, Brazil. Veterinary Parasitology, 124, 1–8. [CrossRef] [PubMed] [Google Scholar]
- Lalošević V, Lalošević D, Čapo I, Simin V, Galfi A, Traversa D. 2013. High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia. Parasite, 20, 3. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. 1998. Dogs are definitive hosts of Neospora caninum. International Journal for Parasitology, 28, 1473–1478. [CrossRef] [PubMed] [Google Scholar]
- McInnes LM, Irwin P, Palmer DG, Ryan UM. 2006. In vitro isolation and characterisation of the first canine Neospora caninum isolate in Australia. Veterinary Parasitology, 137, 355–363. [CrossRef] [PubMed] [Google Scholar]
- MedCalc statistical software 12.6.0 (online version, www.medcalc.org/calc/odds_ratio.php), Ostend, Belgium (accessed 25 August 2013). [Google Scholar]
- Lj Pavičić, Lalošević V, Lj Spasojević-Kosić, Lauš S, Simin S. 2011. Seroprevalence of Neospora caninum in dogs. Contemporary Agriculture, 61, 453–458. [Google Scholar]
- Rinaldi L, Fusco G, Musella V, Veneziano V, Guarino A, Taddei R, Cringoli G. 2005. Neospora caninum in pastured cattle: determination of climatic, environmental, farm management and individual animal risk factors using remote sensing and geographical information systems. Veterinary Parasitology, 128, 219–230. [CrossRef] [PubMed] [Google Scholar]
- Rozsa L, Reiczigel J, Majoros G. 2000. Quantifying parasites in samples of hosts. Journal of Parasitology, 86, 228–232. [Google Scholar]
- Ruehlmann D, Podell M, Oglesbee M, Dubey JP. 1995. Canine neosporosis: a case report and literature review. Journal of the American Animal Hospital Association, 31, 174–183. [PubMed] [Google Scholar]
- Savović M, Lalošević V, Simin S, Pavičić LJ, Boboš S. 2012. Seroprevalence of Neospora caninum in dairy cows with reproductive disorders in Vojvodina province, Serbia. Lucrări Ştiintifice Medicină Veterinară, 45, 161–166. [Google Scholar]
- Statistical Office of the Republic of Serbia, Statistics of Agriculture, Announcement No. 036 released on 21.02.2012, http://webrzs.stat.gov.rs/WebSite/repository/documents/00/00/58/98/po12122011.pdf (accessed 14 May 2013). [Google Scholar]
- Szumilas M. 2010. Explaining Odds ratio. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 19, 227–229. [CrossRef] [Google Scholar]
- Thrusfield M, Ortega C, de Blas I, Noordhuizen JP, Frankena K. 2001. Win Episcope 2.0: improved epidemiological software for veterinary medicine. Veterinary Record, 148, 567–572. [CrossRef] [Google Scholar]
- Vidić B, Grgić Ž, Savić S, Bugarski D. 2011. Neospora caninum causes abortions in cows, Scientific Symposium “Reproduction of domestic animals”, Naučna KMD, Belgrade, Divčibare, Serbia. [Google Scholar]
Cite this article as: Kuruca L, Spasojević-Kosić L, Simin S, Savović M, Lauš & Lalošević V: Neospora caninum antibodies in dairy cows and domestic dogs from Vojvodina, Serbia. Parasite, 2013, 20, 40.
All Tables
Prevalence of seropositive cows according to the geographical origin, farm type and health status.
Prevalence of anti-Neospora caninum antibodies in hunting, stray and farm dogs according to the breed, gender, age and region of origin.
All Figures
 |
Figure 1. Dots on the map of Vojvodina represent localities where blood samples were taken from cows or, in the case of blood collected at the abattoir, places of cows’ origin. Black dots refer to localities where seropositive cows were found, while white dots stand for localities where there were no positive samples. Figures represent the number of sampled cows from specific locality and number of positive ones (between parentheses). |
| In the text | |
 |
Figure 2. Geographical distribution of localities where blood samples were collected from hunting, farm and stray dogs. Numbers outside parentheses represent the dogs from the specific category that was tested on the given locality. Numbers between parentheses refer to the positive ones. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


