| Issue |
Parasite
Volume 20, 2013
|
|
|---|---|---|
| Article Number | 1 | |
| Number of page(s) | 7 | |
| DOI | https://doi.org/10.1051/parasite/2012001 | |
| Published online | 17 January 2013 | |
urn:lsid:zoobank.org:pub:5BEC0C8D-179D-4C59-92B4-8DD30C6B7D56
Research Article
Infective larvae of Cercopithifilaria spp. (Nematoda: Onchocercidae) from hard ticks (Ixodidae) recovered from the Japanese serow (Bovidae)
Larves infectantes de Cercopithifilaria spp. (Nematoda: Onchocercidae) chez des tiques (Ixodidae) récoltées sur des sérows japonais (Bovidae)
1
Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
2
Department of Parasitology, Graduate School of Medicine, Osaka City University, Osaka 545-8585, Japan
3
Muséum National d’Histoire Naturelle, Parasitologie comparée, UMR 7205, CNRS, 75231 Paris, France
4
Mahara Institute of Medical Acarology, Tokushima 779-1510, Japan
5
National Institute of Animal Health, NARO, Ibaraki 305-0856, Japan
6
Research Promotion Project, Oita University, Oita 879-5593, Japan
7
Department of Infectious Disease Control, Faculty of Medicine, Oita University, Oita 879-5593, Japan
* Corresponding author: unishigehiko@um.edu.my
Received:
12
September
2012
Accepted:
19
November
2012
Hard ticks taken from the Japanese serow, Capricornis crispus, in Yamagata Prefecture, Honshu, harboured infective larvae of onchocercid filariae after incubation from the 22nd to the 158th day. Haemaphysalis flava and H. japonica contained one to eight filarial larvae; females, males and a nymph of the ticks were infected. The 44 infective larvae recovered were 612–1,370 μm long, and 11 of them, 930–1,340 μm long, were studied in detail. The larvae possessed the morphologic characteristics of the larvae of the genus Cercopithifilaria, namely an oesophagus with a posterior glandular part, no buccal capsule and a long tail with three terminal lappets. Five types (A to E) of infective larvae were identified based on the morphologic characteristics. While to date five species of Cercopithifilaria have been described from the Japanese serow, a specific identification of the larvae found in this study was generally not possible. Only type E larvae could be tentatively assigned to Cercopithifilaria tumidicervicata, as they had a cervical swelling similar to that of the adults of this species. A key for the identification of the five larval types is presented. The study presents circumstantial evidences indicating that H. flava and H. japonica may transmit Cercopithifilaria spp. to Japanese serows. It also suggests the possibility that such filarial larvae will be found in hard ticks anywhere, because Cercopithifilaria is distributed worldwide, though this genus generally goes unnoticed, as its microfilariae occur in the skin, not in the blood, of host animals.
Résumé
Les tiques récoltées chez les sérows japonais, Capricornis crispus, à Yamagata, Honshu, hébergeaient des larves infectantes de filaires Onchocercidae après incubation du 22ème au 158ème jour. Haemaphysalis flava et H. japonica avaient une à huit larves ; des mâles, des femelles et une nymphe étaient infestés. Les 44 larves infectantes récoltées étaient longues de 612–1,370 μm et 11 d’entre elles, longues de 930 à 1,340 μm, ont été étudiées en détail. Les larves possédaient les caractères morphologiques des larves du genre Cercopithifilaria, c’est-à-dire un œsophage avec une portion postérieure glandulaire, pas de capsule buccale et une longue queue avec 3 languettes terminales. Cinq types de larves infectantes (A à E) ont été identifiés sur la base de caractéristiques morphologiques. Bien que cinq espèces de Cercopithifilaria aient été décrites jusqu’ici chez le sérow japonais, une identification spécifique des larves trouvées dans cette étude n’a généralement pas été possible. Seules les larves de type E ont été identifiées provisoirement à C. tumidicervicata parce qu’elles avaient un renflement cervical comme les adultes de cette espèce. Une clé d’identification des cinq types de larves est présentée. L’étude présente des preuves circonstancielles indiquant que H. flava et H. japonica peuvent transmettre Cercopithifilaria spp. au sérow japonais. Elle suggère la possibilité de trouver partout de telles larves de filaires chez les tiques parce que Cercopithifilaria a une distribution mondiale mais passe généralement inaperçu parce que ses microfilaires sont dans la peau, et non dans le sang.
Key words: Nematoda / Onchocercidae / Cercopithifilaria / Ixodidae / Infective larvae / Japanese serow
© S. Uni et al., published by EDP Sciences, 2013
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cercopithifilaria Eberhard [17] is one of the 94 genera of the family Onchocercidae (Filarioidea). It was created as a subgenus of Dipetalonema Diesing [17] and was soon elevated to generic level [4]. This genus is remarkable for its very large host range and worldwide distribution. The type species was recovered from a cercopithecus monkey in Africa.
Out of a total of 27 nominal species, positively assigned to the genus [9, C. laemmleri (Dasgupta et al. 1978) excluded] only five are parasites of cercopithecus monkeys [10, 11, 17]. Twelve species are parasites of ruminants [4, 13, 16, 19, 20, 34–36], three of rodents [4, 31], one of lagomorphs [12], one of South American didelphid marsupials [18], one of an Australian marsupial [31] and four of carnivores [2, 7, 25, 32]. All these species have the same morphologic characteristics, such as the very tiny buccal capsule (preoesophageal ring), the oesophagus without glandular part and the extremity of the female tail with three lappets, two lateral and one axial [4], or exceptionally reduced to two processes [17].
Up to now the mitochondrial Cox1 and 12S rDNA gene sequences of nine species of Cercopithifilaria were analysed and congeneric species were clustered together [1, 21]. In addition, two unnamed species and Cercopithifilaria grassii (Noè 1907) were identified in European domestic dogs from their microfilariae only, using both morphologic characteristics and molecular analysis; they were grouped with the remaining nominal Cercopithifilaria species [27, 28].
Where data are available, the microfilariae of the species of Cercopithifilaria seem to inhabit the skin. Life cycles of a few species were elucidated in Africa [8, 29], Europe [14, 25, 26, 38] and Australia [30], from a porcupine, dogs, roe deer and a rat. The larval development of the different species of Cercopithifilaria takes place in Ixodidae, which is notable because hard ticks are uncommon intermediate hosts for Onchocercidae. This feature led to the hypothesis that the exceptionally wide geographic distribution and host range of Cercopithifilaria, which suggests many host-switches, might have been facilitated by the hard tick vectors and their peculiar way of life; for instance, their passive long distance transportation, the long lifespan and the feeding cycle that often involves several groups of mammals [7, 28, 34].
In Japan, Cercopithifilaria is present in the black bear and in the two indigenous ruminants, the sika deer and the Japanese serow, that harbour one, two and five species, respectively [32, 34–36]. To investigate their transmission, hard ticks were collected from these mammals and incubated; filarial larvae were recovered [37]. The present study concerns the larvae from ticks taken from the Japanese serow. A morphologic study is essential to confirm that the infective larvae indeed belong to the genus Cercopithifilaria and also to determine if species-specific characteristics are present at the larval stage found in ticks.
Materials and methods
Sixteen serows (Capricornis crispus Temminck, 1845) were killed on Mt. Zao (1,841 m), Yamagata Prefecture, in the northeastern part of Honshu, between April 1998 and July 2001 in accordance with the policies of the Ministry of the Environment, Japan, concerning their conservation and control.
The head with ears, the entire skin of the body with subcutaneous connective tissues and the limbs were shipped refrigerated to the Osaka City University Medical School for examination 1 or 2 days after each animal was killed. Skin snips were made from each serow to determine the presence of filarioids.
Serow identification number: Serows examined for taking skin snips, collecting ticks from the skin and dissecting the carcasses were numbered for identification. Ticks collected from the skin of each carcass were kept in small plastic containers (5 cm in diameter and 7 cm high) with small pores to exchange air and with small pieces of wet filter paper. The containers were placed in a large plastic box with wet tissue paper to prevent desiccation and stored in the incubator (20 °C). Ticks were dissected twice a week from 22nd to 158th day following incubation.
Tick identification number: Ticks harbouring filarial larvae were numbered for identification when the ticks were dissected. The method of tick dissection: A tick was placed in a drop of saline on a glass slide, cut by the disposable scalpels under a dissection microscope. The nematode larvae taken from ticks were fixed in 2% formalin in saline. Ticks were identified by one of us (H.F.) based on the morphologic characteristics [39].
For morphologic studies larvae were cleared in lactophenol and examined under a compound microscope equipped with a camera lucida. Following Bain & Chabaud [6], particular attention was paid to the caudal extremity, and several ratios were calculated: tail length/width at anus (character 1), larval body length (character 2), oesophagus length/body length (character 3; expressed as a percentage), tail length/body length (character 4; expressed as a percentage). These ratios were used to establish the generic morphometric formulae for the infective larvae of Onchocercidae. The genital primordium was examined either at the level of the oesophagus (females) or posterior to the oesophagus (males). Measurements are given in micrometres.
Results
Approximately 2,000 ticks were harvested from 16 serows during the study period. Twenty-two ticks harboured filarial larvae, giving an infection rate of 1%. The number of larvae per tick varied from one to eight (mean 2). The infected ticks were Haemaphysalis flava Newmann, 1897, of which eight females and six males were infected, and Haemaphysalis japonica Warburton, 1908, of which seven females and one nymph were infected (Table 1).
Measurements of infective larvae of Cercopithifilaria spp. recovered from hard ticks collected from the Japanese serow, Capricornis crispus.
Two second-stage larvae were found from ticks: one larva, 337 long and 20 wide, from a tick (Y1: H. japonica, female) dissected at day 22 of incubation after collecting from a serow (YA4); the other larva, 326 long and 21 wide, from a tick (Y2: H. flava, female) dissected at day 24 of incubation after collection from the serow (YA7). The latter larva was found together with seven infective third-stage larvae (612–867 long and 20–26 wide). A total of 44 infective third-stage larvae were recovered, and their body length ranged from 612 to 1,340. From 38 to 158 days of incubation, all larvae recovered had already developed to the infective third-stage. The 11 larvae studied in detail were recovered from six ticks: four H. flava (three males, one female) and two H. japonica (one female, one nymph). The ticks were recovered from five serows and the infective larvae were 940–1,370 long (Table 1). Other measurements were width at midbody, 15–24, total oesophagus length, 267–380 and tail length, 50–75.
In all larvae the cephalic papillae were easily identified but the buccal capsule was inconspicuous (Figure 1A, B, J, N, U). In some larvae a short sclerotized filament protruded from the mouth (Figure 1A, N). The head was rounded (Figure 1B, J, U) or attenuated (Figure 1N). A cephalic swelling was present in two larvae (Figure 1T, arrowhead). The oesophagus had a muscular anterior part and a glandular posterior part with a mosaic appearance (Figure 1A, C, J, N, T). The glandular part was as long as, or longer than the muscular part but not more than twice as long. The glandular part had a constant width (Figure 1C, O) or was attenuated posteriorly (Figure 1T). The excretory cell with a pore at the posterior group of nerve cells was conspicuous (Figure 1A, J, N, T).
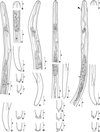 |
Fig. 1. Infective larvae of Cercopithifilaria species from ticks collected from the Japanese serow. (A–F) Type A larva. A. Anterior part, right lateral view. Female genital primordium, arrow. B. Head. C. Oesophageal-intestinal junction. D. Tail, right lateral view at anus. E. Caudal end, ventral view. F. Caudal end, left lateral view. (G–I) Type B larva. G. Tail, right lateral view. H. Caudal end, lateral view. I. Caudal end, ventral view. (J–M) Type C larva. J. Anterior part, right lateral view. K. Tail, left lateral view. L-M. Caudal end, ventral view. (N–S) Type D larva. N. Anterior part, right lateral view. O. Oesophageal-intestinal junction. *Male genital primordium. P. Tail, right lateral view at anus; ventral view at the end. Q. Caudal end, right lateral view. R. Caudal end, ventral view. S. Caudal end, left lateral view. (T–X) Type E larva. T. Anterior part, left lateral view. Cervical swelling, arrowhead; female genital primordium, arrow. U. Head. V. Tail, right lateral view. W. Caudal end, lateral view. X. Caudal end, ventral view. Scale bars: micrometres. |
The tail curved ventrally or was straight with its end attenuated (Figure 1G, P) or truncated (Figure 1D, V). The caudal extremity bore two lateral subterminal lappets (named lappets hereinafter) and an axial terminal lappet (named axial point hereinafter). The lappets were rounded (Figure 1D–F, L, M) or conical (Figure 1G–I, P–S). The width at base was equal to the length (Figure 1H, I) or the base was narrower than the lappet length (Figure 1Q, R). The axial point extended from the tail and was conical (Figure 1G, S), or its base was constricted and its shape rounded (Figure 1D–F), or it was slightly divided (Figure 1X), or it was absent (Figure 1K–M); in this case a ventral transverse crest (or boss) was present (Figure 1K). The genital primordium was found at the level of the glandular oesophagus in the female larvae (Figure 1A, T, arrows) and at the level of the posterior part to the oesophageal-intestinal junction in the male larva (Figure 1O, *).
Five morphologic types of infective larvae were identified by the use of the characteristics described above:
-
Type A (Figure 1A–F): Four larvae, nos. 1–4 (Table 1). Body 1,120–1,370 long, 18–24 wide, oesophagus 267–380; head rounded; tail bent ventrally; tip of tail truncated, axial point constricted at base and rounded; lappets rounded.
-
Type B (Figure 1G–I): One larva, no. 5 (Table 1), 1,250 long, 21 wide; head rounded; tail bent ventrally; tip of tail attenuated, prolonged by conical axial point; lappets conical; width at base of lappets and axial point equal to length.
-
Type C (Figure 1J–M): Two larvae, nos. 6 and 7 (Table 1). Body 970 and 1,340, 19 and 22 wide; head rounded; tail bent ventrally; tip of tail without axial point but with ventral transverse crest (or boss); lappets rounded.
-
Type D (Figure 1N–S): Two larvae, nos. 8 and 9 (Table 1). Body 940 and 1,270 long, 15 and 20 wide; head attenuated anterior to cephalic papillae; tail elongated and straight; tip of tail attenuated, prolonged by conical to elongated axial point; lappets conical to elongated.
-
Type E (Figure 1T–X): Two larvae, nos. 10 and 11 (Table 1). Body 1,091 and 1,190 long, both 18 wide; head rounded; cervical swelling; tail bent ventrally; tip of tail truncated, axial point slightly divided, wide in ventral view and narrow in lateral view; lappets small and rounded. The tick (ID no. Y20) harboured the type E larva (specimen ID no. 10) was taken from the serow (YA2) highly infected with C. tumidicervicata Uni & Bain, 2001.
To facilitate the identification of the infective larvae from ticks taken from serows, a following key is proposed:
-
(2) Cervical swelling; caudal axial point divided; small, round lappets; oesophagus attenuated posteriorly.
Type E larva
-
(1) Without these characteristics.
-
(4) Tail 75 μm.
Caudal axial point and lappets of similar size and conical, elongated shape.
Type D larva
-
(3) Tail 50–60 μm.
-
(6) No caudal axial point, terminal plate with marked ventral crest (or boss), rounded lappets.
Type C larva
-
(5) Axial point present.
-
(8) Axial point constricted at base, of similar shape and size as lappets.
Type A larva
-
(7) Axial point conical.
Type B larva
Discussion
All infective larvae were approximately 1 mm long and morphologically similar. They possessed an oesophagus with a glandular posterior part, which marked them as belonging to the Onchocercidae [5]. They had a long tail and caudal lappets like Acanthocheilonema Cobbold, 1870 [33] and several other closely related genera that had previously been placed in the Dipetalonema “lineage” [3, 4, 15] but they lacked the buccal capsule. In this they resembled the infective larvae of the species of Cercopithifilaria [6]. The larvae were therefore assigned to the latter genus without any doubt.
However, the morphometric formula established by Bain & Chabaud [6], based on three species parasitic in roe deer, dogs and porcupines, respectively, must be slightly amended. The eight larvae were shorter than 1,300 and three larvae were longer than 1,300 (Table 1); the character 2 (body length): 2B (>1,300) is changed into 2X (X indicates the length between 800 and 1299) and 2B. The characters 1 (tail length/width at anus, 3.0 to 5.8: 1B) and 3 (oesophagus/body length, smaller than 39%: 3A) are confirmed, whereas character 4 (tail/body length, 4.0–5.9%: 4X) is at present 3.9–8.0%: 4X with minor variation and 4B). The original formula, 1B, 2B, 3A, 4X, is therefore changed to 1B, 2X and 2B, 3A, 4X and 4B.
In the set of 11 larvae that were examined in detail, several species seemed to be present since the morphologic characteristics allowed us to distinguish five types of larvae. Attempts to relate each type to one of the five species of Cercopithifilaria parasitizing the serows must be made with caution. Firstly, the ticks collected from the serows may contain larvae from other hosts also infected with Cercopithifilaria, such as sika deer and black bears [32, 34; ongoing work]. Both H. flava and H. japonica are three-host ticks that require three kinds of host animals in their life cycle. The larvae of H. flava are often found on the skin of hares and the adults parasitize large size mammals such as deer, serows and bears on the Japanese islands, including Okinawa & Hokkaido [22]. The tick also is found in the Russian Far East and China [24]. The larvae and adults of H. japonica parasitize wild mammals such as hares, serows, deer and black bears on the western and northern parts of Honshu, Japan [39].
Our study indicates that the ticks, H. flava and H. japonica, are possible intermediate hosts of Cercopithifilaria spp. of serows in Japan; many more larvae were found from H. flava than H. japonica. We estimate that microfilariae of Cercopithifilaria spp. from serows need to develop in a female of H. flava to infective stage in 24 days or more at 20 °C. We found that a nymph of H. japonica harboured larvae of Cercopithifilaria spp. The finding suggests trans-stadial transmission of the filarial larva if a larva had molted into the nymph during incubation.
Secondly, while the caudal extremities of adult females also bear lappets and axial points that differ between species [34–36], the detailed morphology of these structures has to be compared with that seen in infective larvae; similarly to several infective larvae (types A and C), female adults of C. bulboidea Uni & Bain, 2001 and C. shohoi Uni et al., 1998 present lappets or axial points that are constricted at the base and rounded [35, 36]. While the female adults of C. minuta Uni & Bain, 2001 present conical and acute lappets and axial point viewed by a scanning electron microscope [35], the features appear to be similar to those of the type D larva.
Thirdly, the extent of intraspecific variation of minor features of the caudal extremity of infective larvae is at present unknown. Only type E could be tentatively identified as C. tumidicervicata based on the features of the anterior part and the tail end. Adults of this species show particular characteristics, such as a cervical swelling, a truncated tail end and a slightly bifid axial point [35]. Such characteristics were found in the type E larvae (Figure 1T, V–X). Cercopithifilaria tumidicervicata is found from serows in Yamagata Prefecture, together with C. shohoi and C. minuta [35]. The larva (specimen ID no. 10) of the type E was found from the tick taken from the serow highly infected with C. tumidicervicata (Table 1).
The role of hard ticks in the transmission of Cercopithifilaria species and in host-switches during their evolution is once more supported by this study. Several genes of the Cercopithifilaria species from the serow and other hosts have been sequenced [1, 21, 27, 28]. The present morphologic analysis will assist in future attempts to identify specimens to species level using gene sequencing, as done by Brianti et al. [14] with the Cercopithifilaria species of dogs and in the genus Onchocerca by Fukuda et al. [23].
Acknowledgments
We thank Professors Dr. Mohd Sofian Bin Azirun, Dean of the Faculty of Science, University of Malaya, Dr. Rosli Bin Hashim, Head of the Institute of Biological Sciences, Faculty of Science, University of Malaya, and Dr. Rosli Bin Ramli of the same institute, who supported our study. This work was partly supported by the Ministry of Higher Education, Malaysia (FRGS FP020-2012).
In memoriam
With great sorrow, we have learned that our esteemed, distinguished co-author, Professor Odile Bain, passed away on 16 October 2012, after this article was submitted to Parasite. We shall always be deeply grateful for her kind, dedicated support of studies on filarial parasites in Japan.
References
- Agatsuma, T, Iwagami M, Uni S, Takaoka H, Katsumi A, Kimura E, and Bain O. 2005. Molecular phylogenetic relationships among seven Japanese species of Cercopithifilaria. Parasitology International, 54, 195–199. [CrossRef] [PubMed] [Google Scholar]
- Almeida GLG, and Vicente JJ. 1984. Cercopithifilaria bainae sp. n. parasita de Canis familiaris (L.) (Nematoda, Filarioidea). Atas Sociedade de Biologia do Rio de Janeiro, 24, 18. [Google Scholar]
- Anderson RC, and Bain O. 1976. Keys to genera of the order Spirurida. Part 3. Diplotriaenoidea, Aproctoidea and Filarioidea, in CIH keys to the nematode parasites of vertebrates, Anderson RC, Chabaud AG, and Willmott S, Editors. No. 3, Archival volume 2009. CAB International: Oxfordshire, UK, pp. 59–116. [Google Scholar]
- Bain O, Baker M, Chabaud AG. 1982. Nouvelles données sur la lignée Dipetalonema (Filarioidea, Nematoda). Annales de Parasitologie Humaine et Comparée, 57, 593–620. [Google Scholar]
- Bain O, Casiraghi M, Martin C, and Uni S. 2008. The Nematoda Filarioidea: critical analysis linking molecular and traditional approaches. Parasite, 15, 342–348. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Bain O, Chabaud AG. 1986. Atlas des larves infestantes de filaires. Tropical Medicine and Parasitology, 37, 301–340. [Google Scholar]
- Bain O, Chabaud AG. and Georges AJ. 1988. Nouvelle filaire du genre Cercopithifilaria, parasite d’un carnivore africain. Parassitologia, 29, 63–69. [Google Scholar]
- Bain O, Petit G, Chabaud AG. 1986. Une nouvelle filaire, Cercopithifilaria roussilhoni n. sp., parasite de l’Athérure au Gabon, transmise par tiques: hypothèse sur l’évolution du genre. Annales de Parasitologie Humaine et Comparée, 61, 81–93. [Google Scholar]
- Bain O, Uni S, Takaoka H. 2002. A synthetic look at a twenty year old taxon, Cercopithifilaria; its probable evolution. Proceedings of the 10th International Congress of Parasitology, Monduzzi Editore, Milan, 365–368. [Google Scholar]
- Bain O. Wamae CN, Reid GDF. 1988. Diversité des filaires du genre Cercopithifilaria chez les babouins, au Kenya. Annales de Parasitologie Humaine et Comparée, 63, 224–239. [Google Scholar]
- Bain O, Wamae CN, Reid GDF. 1989. Description de Cercopithifilaria verveti n. sp., filaire sous-cutanée d’un cercopithèque au Kenya. Annales de Parasitologie Humaine et Comparée, 64, 42–45. [Google Scholar]
- Bartlett CM. 1983. Cercopithifilaria leporinus n. sp. (Nematoda: Filarioidea) from the snowshoe hare (Lepus americanus Erxleben) (Lagomorpha) in Canada. Annales de Parasitologie Humaine et Comparée. 58, 275–283. [Google Scholar]
- Böhm LK, Supperer R. 1953. Beobachtungen über eine neue Filarie (Nematoda), Wehrdickmansia rugosicauda Böhm & Supperer 1953, aus dem subkutanen Bindegewebe des Rehes. Sitzungsberichte, Abt. 1, Biologie, Mineralogie, Erdkunde und verwandte Wissenschaften. Österreichische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, 162, 95–103. [Google Scholar]
- Brianti E, Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, Gaglio G, Napoli E, Brucato G, Cauquil L, Giannetto S, Bain O. 2012. Rhipicephalus sanguineus (Ixodida, Ixodidae) as intermediate host of a canine neglected filarial species with dermal microfilariae. Veterinary Parasitology, 183, 330–337. [CrossRef] [PubMed] [Google Scholar]
- Chabaud AG, Bain O. 1976. La lignée Dipetalonema. Nouvel essai de classification. Annales de Parasitologie Humaine et Comparée, 51, 365–397. [Google Scholar]
- Chabaud AG, Landau I, Petit G. 1978. Deux Filaires de Céphalophes au Gabon. Annales de Parasitologie Humaine et Comparée, 53, 285–290. [Google Scholar]
- Eberhard ML. 1980. Dipetalonema (Cercopithifilaria) kenyensis subgen. et sp. n. (Nematoda: Filarioidea) from African baboons, Papio anubis, Journal of Parasitology, 66, 551–554. [CrossRef] [Google Scholar]
- Esslinger JH, Smith JL. 1979. Dipetalonema (Acanthocheilonema) didelphis sp. n. (Nematoda: Filarioidea) from opossums, with a redescription of D. (A.) pricei (Vaz and Pereira, 1934). Journal of Parasitology, 65, 928–933. [CrossRef] [Google Scholar]
- Fain A. 1977. Parasitisme intradermique par les Nématodes chez les bovins au Rwanda. Description de deux nouvelles espèces, Annales de la Société belge de médecine tropicale, 57, 113–120. [Google Scholar]
- Fain A, Hérin V. 1955. Filarioses des bovidés au Ruanda-Urundi. III. Étude parasitologique. Annales de la Société belge de médecine tropicale, 35, 535–554. [Google Scholar]
- Ferri E, Barbuto M, Bain O, Galimberti A, Uni S, Guerrero RA, Ferté H, Bandi C, Martin C, Casiraghi M. 2009. Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda), Frontiers in Zoology, 6, 1 doi: 10.1186/1742-9994-6-1 [CrossRef] [PubMed] [Google Scholar]
- Fujita H, Tsuboi Y. 2000. A survey of ixodid ticks (Acari: Ixodidae) including a list of all known species in Okinawajima Island, Japan. Journal of the Acarological Society of Japan, 9, 45–49. [CrossRef] [Google Scholar]
- Fukuda M, Otsuka Y, Uni S, Bain O, Takaoka H. 2010. Molecular identification of infective larvae of three species of Onchocerca found in wild-caught females of Simulium bidentatum in Japan. Parasite, 17, 39–45. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Kitaoka S. 1985. Keys to the species in immature stages of the Japanese Haemaphysalis ticks (Ixodidae). Bulletin of the National Institute of Animal Health, 88, 49–63. [Google Scholar]
- Noè G. 1907. Contribuzione alla sistematica ed alla anatomia del genera Filaria. 1. La Filaria grassii (Noè, 1907). Richerche del Laboratorio di Anatomia Normale delle Regia Università di Roma, 15, 235–252. [Google Scholar]
- Noè G. 1908. Il ciclo evolutivo della Filaria grassii, mihi, 1907. Atti dell’ Accademia Nationale dei Lincei Rendiconti, Classe de Scienze Fisiche Matematiche e Naturali, 17, 282–293. [Google Scholar]
- Otranto D, Brianti E, Dantas-Torres F, Miró G, Latrofa MS, Mutafchiev Y, Bain O. 2012. Species diversity of dermal microfilariae of the genus Cercopithifilaria infesting dogs. Parasitology, doi: 10.1017/S0031182012001357 [Google Scholar]
- Otranto D, Brianti E, Dantas-Torres F, Weigla S, Latrofa MS, Gaglio G, Cauquil L, Giannetto S, Bain O. 2011. Morphological and molecular data on the dermal microfilariae of a species of Cercopithifilaria from a dog in Sicily. Veterinary Parasitology, 182, 221–229. [CrossRef] [PubMed] [Google Scholar]
- Petit G, Bain O, Cassone J, Seureau C. 1988. La filaire Cercopithifilaria roussilhoni chez la tique vectrice. Annales de Parasitologie Humaine et Comparée, 63, 296–302. [Google Scholar]
- Spratt DM, Haycock P. 1988. Aspects of the life-history of Cercopithifilaria johnstoni (Nematoda: Filarioidea). International Journal for Parasitology, 18, 1087–1092. [CrossRef] [PubMed] [Google Scholar]
- Spratt DM, Varughese G. 1975. A taxonomic revision of filarioid nematodes from Australian marsupials. Australian Journal of Zoology, Suppl. Ser., 35, 1–99. [CrossRef] [Google Scholar]
- Uni S. 1983. Filarial parasites from the black bear of Japan. Annales de Parasitologie Humaine et Comparée, 58, 71–84. [Google Scholar]
- Uni S, Bain O, Suzuki K, Agatsuma T, Harada M, Motokawa M, Martin C, Lefoulon E, Fukuda M, Takaoka H. 2013. Acanthocheilonema delicata n. sp. (Nematoda: Filarioidea) from Japanese badgers (Meles anakuma): Description, molecular identification, and Wolbachia screening. Parasitology International, 62, 14–23. [CrossRef] [PubMed] [Google Scholar]
- Uni S, Bain O, Takaoka H, Katsumi A, Fujita H, Suzuki Y. 2002. Diversification of Cercopithifilaria species (Nematoda: Filarioidea) in Japanese wild ruminants with description of two new species. Parasite, 9, 293–304. [PubMed] [Google Scholar]
- Uni S, Suzuki Y, Baba M, Mitani N, Takaoka H, Katsumi A, Bain O. 2001. Coexistence of five Cercopithifilaria species in the Japanese rupicaprine bovid, Capricornis crispus, Parasite, 8, 197–213. [PubMed] [Google Scholar]
- Uni S, Suzuki Y, Katsumi A. 1998. Cercopithifilaria shohoi n. sp. (Nematoda: Filarioidea) from the relict Bovidae, Capricornis crispus, in Japan. Parasite, 5, 119–126. [PubMed] [Google Scholar]
- Uni S, Suzuki Y, Katsumi A, Kimata I, Iseki M, Bain O. 1998. Taxonomy and pathology of filarial parasites from Japanese serows (Capricornis crispus). Proceedings of the 4th International Congress of Parasitology, Monduzzi Editoriale: Milano. 681–684. [Google Scholar]
- Winkhardt HJ. 1980. Untersuchungen über den Entwicklungszyklus von Dipetalonema rugosicauda (syn. Wehrdikmansia rugosicauda) (Nematoda: Filarioidea). II. Die Entwicklung von Dipetalonema rugosicauda im Zwischenwirt Ixodes ricinus und Untersuchungen über das Vorkommen der Mikrofilarien im Reh (Capreolus capreolus). Tropenmedizin und Parasitologie, 31, 21–30. [PubMed] [Google Scholar]
- Yamaguti N, Tipton VJ, Keegan HL, Toshioka S. 1971. Ticks of Japan, Korea, and the Ryukyu Islands. Brigham Young University Science Bulletin, Biological series, 15, 1–226. [Google Scholar]
Cite this article as: Uni S, Bain O, Fujita H, Matsubayashi M, Fukuda M & Takaoka H: Infective larvae of Cercopithifilaria spp. (Nematoda: Onchocercidae) from hard ticks (Ixodidae) recovered from the Japanese serow (Bovidae). Parasite, 2013, 20, 1.
All Tables
Measurements of infective larvae of Cercopithifilaria spp. recovered from hard ticks collected from the Japanese serow, Capricornis crispus.
All Figures
 |
Fig. 1. Infective larvae of Cercopithifilaria species from ticks collected from the Japanese serow. (A–F) Type A larva. A. Anterior part, right lateral view. Female genital primordium, arrow. B. Head. C. Oesophageal-intestinal junction. D. Tail, right lateral view at anus. E. Caudal end, ventral view. F. Caudal end, left lateral view. (G–I) Type B larva. G. Tail, right lateral view. H. Caudal end, lateral view. I. Caudal end, ventral view. (J–M) Type C larva. J. Anterior part, right lateral view. K. Tail, left lateral view. L-M. Caudal end, ventral view. (N–S) Type D larva. N. Anterior part, right lateral view. O. Oesophageal-intestinal junction. *Male genital primordium. P. Tail, right lateral view at anus; ventral view at the end. Q. Caudal end, right lateral view. R. Caudal end, ventral view. S. Caudal end, left lateral view. (T–X) Type E larva. T. Anterior part, left lateral view. Cervical swelling, arrowhead; female genital primordium, arrow. U. Head. V. Tail, right lateral view. W. Caudal end, lateral view. X. Caudal end, ventral view. Scale bars: micrometres. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


