Figure 2
Download original image
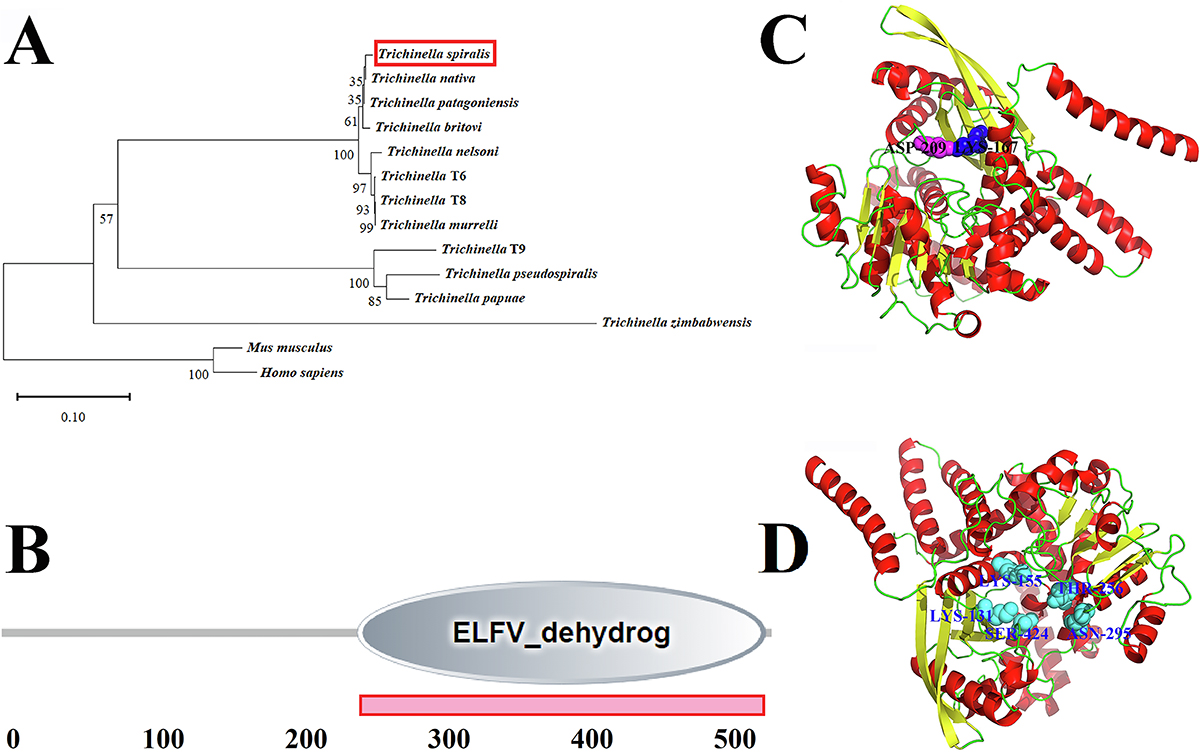
Phylogenetic tree construction, prediction of function domain, and tertiary structure of TsGDH. A: TsGDH in the evolutionary tree of Trichinella, humans and the mouse. The evolutionary tree of glutamate dehydrogenase of 12 different species/genotypes of the genus Trichinella was constructed by the neighbor-joining (NJ) method. B: TsGDH has an ELFV_dehydrog domain at 249-535 aa. C: Prediction of the tertiary structure of TsGDH. The homologous modeling and prediction of the tertiary structure of TsGDH were achieved using Alphafold2. TsGDH has one enzyme active site (Lys 167) displayed as a dark blue spherical shape, and one catalytic site (Asp 209) displayed as a purple spherical shape. D: The substrate binding sites of TsGDH are displayed in a light blue spherical shape, including Lys 131, Lys 155, Thr 256, Asn 295, and Ser 424.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


