| Issue |
Parasite
Volume 30, 2023
|
|
|---|---|---|
| Article Number | 53 | |
| Number of page(s) | 15 | |
| DOI | https://doi.org/10.1051/parasite/2023054 | |
| Published online | 08 December 2023 | |
urn:lsid:zoobank.org:pub:ABB00F93-232A-4551-930D-DBEFDCE602C8
Research Article
Dactylogyrids (Platyhelminthes, Monogenea) from the gill lamellae of doradids (Siluriformes) with description of five new species of Cosmetocleithrum and new geographical distribution for known species from the Neotropical Region, Brazil
Dactylogyridae (Platyhelminthes, Monogenea) des lamelles branchiales de Doradidae (Siluriformes) avec description de cinq nouvelles espèces de Cosmetocleithrum et nouvelle répartition géographique d’espèces connues de la région néotropicale au Brésil
1
Programa de Pós-graduação em Ciência Animal, Laboratório de Multiusuários em Pesquisa da Pós-graduação (LAMP) – Universidade Estadual do Maranhão (UEMA). Cidade Universitária Paulo VI, Avenida Lourenço Vieira da Silva, 1000, São Luís, Maranhão, MA, Brazil
2
Laboratório de Helmintos Parasitos de Peixes, Instituto Oswaldo Cruz, FIOCRUZ, Av. Brasil, 4365, Rio de Janeiro, RJ 21045-900, Brazil
3
Laboratório de Biologia Geral do Instituto Federal de Acre (IFAC), Campus Cruzeiro do Sul, Estrada da Apadec no. 1192, Bairro Nova Olinda, CEP: 69980-000, Cruzeiro do Sul, Acre, Brazil
4
Laboratorio de Parasitologia e Doenças Parasitárias dos Animais – LPDP, UEMA, Cidade Universitária Paulo VI, Avenida Lourenço Vieira da Silva, 1000, São Luís, Maranhão, MA, Brazil
* Corresponding author: scohen@ioc.fiocruz.br; cohen.simone@gmail.com
Received:
10
October
2023
Accepted:
12
November
2023
Five new species of Cosmetocleithrum were described parasitizing the gill filaments of neotropical doradid fishes. Cosmetocleithrum undulatum n. sp., Cosmetocleithrum brachylecis n. sp. and Cosmetocleithrum ludovicense n. sp. are described from Platydoras brachylecis from a market-place of São Luís, State of Maranhão, Brazil. Cosmetocleithrum sacciforme n. sp. and Cosmetocleithrum basicomplexum n. sp. are described from Oxydoras niger from Juruá River, State of Acre, Brazil. Cosmetocleithrum undulatum and Cosmetocleithrum brachylecis resemble Cosmetocleithrum falsunilatum Feronato, Razzolini, Morey & Boeger, 2022 mainly by the unique male copulatory organ (MCO) morphology but differ from these and all congeneric species mainly by the morphology of the MCO, accessory piece and hooks pairs. Cosmetocleithrum ludovicense is closer to Cosmetocleithrum confusus Kritsky, Thatcher & Boeger, 1986 and to Cosmetocleithrum akuanduba Soares, Santos Neto & Domingues, 2018 but differs from those mainly by the morphology of the accessory piece. Cosmetocleithrum sacciforme differs from all congeneric species mainly by the morphology of the accessory piece formed by a single plate of saccular appearance. Cosmetocleithrum basicomplexum also shares morphological characters with Cosmetocleithrum gigas Morey, Cachique & Babilonia, 2019 considering the size of the body and shape of the anchors, but differs mainly in the morphology of the bars and hooks. Besides the new species, new data are presented for Cosmetocleithrum leandroi Soares, Neto & Domingues, 2018, C. akuanduba and C. confusus regarding morphological characteristics and biogeography.
Résumé
Cinq nouvelles espèces de Cosmetocleithrum sont décrites, parasitant les filaments branchiaux de poissons Doradidae néotropicaux. Cosmetocleithrum undulatum n. sp., Cosmetocleithrum brachylecis n. sp. et Cosmetocleithrum ludovicense n. sp. sont décrits de Platydoras brachylecis provenant d’un marché de São Luís, État du Maranhão, Brésil. Cosmetocleithrum sacciforme n. sp. et Cosmetocleithrum basicomplexum n. sp. sont décrits d’Oxydoras niger de la rivière Juruá, État d’Acre, Brésil. Cosmetocleithrum undulatum et Cosmetocleithrum brachylecis ressemblent à Cosmetocleithrum falsunilatum Feronato, Razzolini, Morey & Boeger, 2022 principalement par la morphologie unique de l’organe copulateur mâle (OCM), mais diffèrent de ces espèces et de toutes les espèces congénères principalement par la morphologie de l’OCM, de la pièce accessoire et des paires de crochets. Cosmetocleithrum ludovicense est proche de Cosmetocleithrum confusus Kritsky, Thatcher & Boeger, 1986 et de Cosmetocleithrum akuanduba Soares, Santos Neto & Domingues, 2018 mais en diffère principalement par la morphologie de la pièce accessoire. Cosmetocleithrum sacciforme se distingue de toutes les espèces congénères principalement par la morphologie de la pièce accessoire formée d’une seule plaque d’aspect sacculaire. Cosmetocleithrum basicomplexum partage également des caractères morphologiques avec Cosmetocleithrum gigas Morey, Cachique & Babilonia, 2019 compte tenu de la taille du corps et de la forme des anchors, mais en diffère principalement par la morphologie des barres et des crochets. Outre les nouvelles espèces, de nouvelles données sont présentées pour Cosmetocleithrum leandroi Soares, Neto & Domingues, 2018, C. akuanduba et C. confusus concernant les caractéristiques morphologiques et la biogéographie.
Key words: Cosmetocleithrum / Dactylogyridae / Monogeneans / Doradidae / South America
© A.L. Silva et al., published by EDP Sciences, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The neotropics, spanning from central Mexico to the southern limit of South America, has the most diverse group of fishes on the planet [14]. This region harbors the greatest diversity of freshwater fish with approximately 6,000 known species and estimates of 9,000 species [18]. Of the total known species, the Characiformes, Siluriformes, and Gymnotiformes account for approximately 77% of the known species [2]. Thus, South America harbors the most diverse fauna of continental freshwater fish in the world with approximately 5,750 known species [3]. Siluriformes, collectively known as catfish, stand out as the largest and most diverse order of freshwater fish, and constitute one of the most important components of the Neotropical fauna, with more than 3,800 described species [6]. Among all the families of this order, Doradidae stand out as one of the most diverse and representative families among the Neotropical Siluriformes, with more than 90 valid species [6]. Doradidae are a monophyletic group endemic to freshwaters of South America on both sides of the Andes Mountains [19].
Siluriformes host a remarkably rich and diverse fauna of gill monogeneans, and these host-parasite systems are an attractive model for phylogenetic studies in the Neotropics [4, 12]. The dactylogyrid Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986 is one of the most species-rich groups of monogenoids reported from siluriform fishes and was proposed to accommodate species of Dactylogyridae that parasitize Oxydoras niger (Valenciennes) and Pterodoras granulosus (Valenciennes) in the Amazon River basin [9]. This genus shows high specificity to catfishes within Doradidae and Auchenipteridae [11]. Currently, 23 species of Cosmetocleithrum are recognized as parasites of the gills of neotropical Siluriformes, among which 15 species recorded in doradids hosts, eight in Auchenipteridae, one parasitizing Pimelodidae, and one in Loricariidae. All species of Cosmetocleithrum have been described from hosts of members of a single family [1, 4, 5, 15, 22, 23, 24], except for Cosmetocleithrum bulbocirrus Kritsky, Thatcher & Boeger, 1986, reported from species of three different siluriform families and from Hoplias malabaricus (Bloch), a characiform fish [7]. However, considering that only one specimen was found in the latter host, this record needs to be confirmed.
During research on monogeneans of siluriform fishes in northern and northeastern Brazil, five new species of Cosmetocleithrum were found and are described herein. Moreover, new data are presented to Cosmetocleithrum leandroi Soares, Neto & Domingues, 2018, Cosmetocleithrum akuanduba Soares, Santos Neto & Domingues, 2018 and Cosmetocleithrum confusus Kritsky, Thatcher & Boeger, 1986 regarding morphological characteristics and biogeographical data, thus expanding the knowledge of these species.
Material and methods
The studies were carried out between 2019 and 2023 on different doradid species collected from two distinct localities in Brazil. Specimens of Oxydoras niger were captured with gill nets and hook and line from Juruá River, Acre, Brazil (7°40′34.1″S, 72°39′39.5″W) and those from Platydoras brachylecis and Hassar affinis were obtained from street markets located on the island of São Luís, State of Maranhão (2°34′18.0″S 44°11′49.9″W).
The gills of each specimen were removed and placed in vials containing hot water (c. 65 °C) in order to relax the parasites, and they were then shaken to detach the parasites from the gill filaments. Subsequently, absolute ethanol was added to reach a concentration of 70%. The vials were then sent to “Laboratório de Helmintos Parasitos de Peixes, Instituto Oswaldo Cruz, FIOCRUZ”, where the gills were analyzed, and the parasites identified. Monogeneans were picked using a stereoscopic microscope for subsequent morphological studies. Some specimens were mounted in Hoyer’s medium for study of the sclerotized parts; others were stained with Gomori’s trichrome for study of the internal organs of the parasite [8]. Measurements are presented in micrometers; range values are followed by mean and number of structures measured in parentheses. Dimensions of organs and other structures represent the greatest distance; lengths of curved or bent structures (anchors, bars, and accessory piece) represent the straight-line distances between extreme ends [9], except for copulatory complexes that were measured using ImageJ [17]. The specimens were studied, photographed, and drawn using an Olympus BX 41 microscope with phase contrast and Zeiss Axioskop 2 Plus microscope with differential interference contrast (DIC), both equipped with a camera lucida. Holotypes, paratypes, and vouchers of each species were deposited in the “Coleção Helmintológica do Instituto Oswaldo Cruz - CHIOC”, from “Fundação Oswaldo Cruz - FIOCRUZ”.
Results
Class Monogenea Bychowsky, 1937
Subclass Monopisthocotylea Odhner, 1912
Order Dactylogyridea Bychowsky, 1937
Dactylogyridae Bychowsky, 1933
Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986
Cosmetocleithrum undulatum n. sp. (Fig. 1)
urn:lsid:zoobank.org:act:D915B062-6777-4D6F-8024-C5CF6904F062
 |
Figure 1 Cosmetocleithrum undulatum n. sp. from Platydoras brachylecis. (A) Total ventral view (composite); (B) Copulatory complex, ventral view; (C) Hook pairs 1–4, 6, 7; (D) Hook pair 5; (E) Dorsal bar; (F) Ventral bar; (G) Ventral anchor; (H) Dorsal anchor. Scale bars: A, 100 μm, B, C, 10 μm, D, 50 μm, E, F, 15 μm, G, H, 20 μm. |
Type-host: Platydoras brachylecis Piorski, Garavello, Arce H & Sabaj Pérez, 2008 (Siluriformes, Doradidae).
Site in host: Gill lamellae.
Type-locality: Market-place of the Cidade Operária, São Luís, State of Maranhão, Brazil (2°34′18.0″S 44°11′49.9″W).
Parasitological indexes: Total number of hosts: 3; number of infected hosts: 3; total number of parasites: 312.
Type-material: Holotype CHIOC 40257 a; Paratypes CHIOC 40257 b–h; 40258 a–g.
Etymology: The specific name is derived from Latin (undulatum = wavy) and refers to the shape of the male copulatory organ.
Description
Based on 21 specimens: 14 mounted in Hoyer’s medium and 7 stained in Gomori’s trichrome. Body fusiform, elongated, comprising cephalic region, trunk, peduncle, and haptor. Tegument thin, smooth. Body, including haptor, 1069 (780–1380; n = 14) long by 228 (110–320; n = 14) wide at the level of germarium. Cephalic lobes poorly developed. Four pairs of head organs. Cephalic glands indistinct. Eyes absent. Accessory granules scattered in the pharyngeal region, sometimes gathered, resembling eyes. Mouth subterminal. Pharynx spherical 52 (32–73; n = 7) in diameter. Esophagus short. Two intestinal ceca confluent posteriorly to testis, lacking diverticula. Gonads intercecal, tandem. Germarium 97 (83–125; n = 4) long by 21 (14–36; n = 4) wide. Vagina simple, non-sclerotized; vaginal aperture sinistroventral. Seminal receptacle pyriform. Mehlis’ gland, uterus, oviduct, and ootype not observed. Vitellaria extends throughout the trunk, except in areas of other reproductive organs. Testis postgermarial, 49 (32–60; n = 3) long by 20 (15–30; n = 3) wide. Vas deferens looping left intestinal cecum; seminal vesicle a dilatation of vas deferens. Prostatic reservoir elongated. Copulatory complex comprising male copulatory organ (MCO) and accessory piece. MCO formed by a wavy tube and sclerotized walls that abruptly tapers at the tip; a thin, and delicate veil-shaped layer covers the entire tip of the cirrus and involves the accessory piece in its distal portion, 220 (200–249; n = 14) in total length. Accessory piece straight, non-articulated to the base of the MCO, 98 (86–116; n = 14) long. Peduncle conspicuous, long. Haptor globose, with dorsal and ventral anchor/bar complexes, 177 (110–220; n = 14) wide. Ventral bar straight with enlarged ends projecting posteriorly, 57 (49–72; n = 14) wide. Dorsal bar more straight with two submedian projections, 69 (57–85; n = 14) wide. Ventral and dorsal anchors dissimilar in size and shape. Ventral anchor with well-developed, square-shaped superficial root and rounded deep root, curved shaft and point not passing from the level of the tip of superficial root, outer 59 (55–61; n = 14); inner 50 (47–54; n = 14); base 33 (29–40; n = 14). Dorsal anchor with well-developed rectangular superficial root and well-developed and rounded deep root, evenly curved shaft and point not passing from the level of the tip of superficial root, outer 41 (39–43; n = 14); inner 37 (35–43; n = 14); base 26 (22–30; n = 14). Hooks with ancyrocephaline distribution. Hooks dissimilar, pair 5 with a delicate point, inconspicuous thumb, slender straight shank with point slightly recurved, filamentous hook (FH) loop 3/4 shank length, 18 (17–19; n = 13); hook pairs 1–4, 6 and 7 with shaft powerfully robust through its length, ending in a small rounded portion at the end; thumb short, rounded; filamentous hook (FH) loop about shank length, 16 (15–18; n = 78).
Remarks
Cosmetocleithrum undulatum n. sp. differs from all congeneric species mainly in terms of the morphology of the MCO, while resembling C. falsunilatum Feronato, Razzolini, Morey & Boeger, 2022 with a unique MCO morphology, very similar to the species of Unilatus Mizelle & Kritsky, 1967, that are parasites of loracariids. However, Cosmetocleithrum undulatum n. sp. differs from C. falsunilatum regarding the shape of the MCO, which in C. falsunilatum is rolled up into a corkscrew shaped, while in Cosmetocleithrum undulatum n. sp. the MCO is an undulating tube with a thin and delicate layer that covers the entire tip of the MCO. Furthermore, the accessory piece of C. falsunilatum is subdivided into two regions that reach close to the base of the MCO, while in the new species, the accessory piece is straight, reaching just less than half of the length of the MCO. The hook pairs 1–4, 6, 7 of the new species are unique among the Cosmetocleithrum species, with a shaft that is strongly robust throughout its length, and with a small constricted portion ending with a small rounded portion at the end.
Cosmetocleithrum brachylecis n. sp. (Fig. 2)
urn:lsid:zoobank.org:act:D915B062-6777-4D6F-8024-C5CF6904F062
 |
Figure 2 Cosmetocleithrum brachylecis n. sp. from Platydoras brachylecis. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Hook; (D) Dorsal bar; (E) Ventral bar; (F) Ventral anchor; (G) Dorsal anchor. Scale bars: A, 100 μm, B, D–G, 20 μm, C, 10 μm. |
Type-host: Platydoras brachylecis Piorski, Garavello, Arce H & Sabaj Pérez, 2008 (Siluriformes, Doradidae).
Site in host: Gill lamellae.
Type-locality: Market-place of the Cidade Operária, São Luís, State of Maranhão, Brazil (2°34′18.0″S 44°11′49.9″W).
Parasitological indexes: Total number of hosts: 3; number of infected hosts: 3; total number of parasites: 38.
Type-material: Holotype CHIOC 40247 a; Paratypes CHIOC 40247 b–m; 40248.
Etymology: The specific name is derived from the name of its host, Platydoras brachylecis.
Description
Based on 16 specimens mounted in Hoyer’s medium and 3 stained in Gomori’s trichrome. Body fusiform, elongated, comprising cephalic region, trunk, peduncle, and haptor. Body, including haptor, 528 (360–660; n = 13) long by 116 (60–160; n = 13) wide at the germarium level. Tegument thin, smooth. Cephalic lobes poorly developed. Four pairs of head organs. Cephalic glands indistinct. Eyes absent. Accessory granules scattered in the cephalic and pharyngeal region. Mouth subterminal. Pharynx spherical, muscular 34 (27–43; n = 9) in diameter. Esophagus short. Two intestinal ceca confluent just posteriorly to gonads, lacking diverticula. Gonads tandem, germarium pre-testicular. Germarium 110 (72–157; n = 4) long by 23 (17–23; n = 4) wide. Vagina simple, non-sclerotized; vaginal aperture sinistroventral. Seminal receptacle rounded. Mehlis’ gland, uterus, oviduct and ootype not observed. Vitelline follicles dense, dispersed throughout trunk but absent in region of reproductive organs and MCO. Testis posterior to germarium 27 (22–32; n = 4) long by 27 (22–35; n = 4) wide. Vas deferens looping left intestinal cecum; seminal vesicle a dilatation of vas deferens. Prostatic reservoir anterior to seminal vesicle. Copulatory complex comprising male copulatory organ (MCO) and accessory piece. MCO composed as a spiral tube with sclerotized walls, with counter-clockwise orientation, 68 (49–82; n = 13) in total length. Accessory piece straight, elongated, exceeding the base of the cirrus, non-articulated to the base, 45 (36–52; n = 13) long. Peduncle elongate. Haptor globose, almost the same width as the body 104 (60–130; n = 13) wide. Ventral bar slightly recurved with enlarged ends, 38 (24–43; n = 13) wide. Dorsal bar robust, broadly u-shaped, with two submedian projections, 46 (25–55; n = 13) wide. Ventral and dorsal anchors dissimilar in size and similar in shape, robust superficial and deep roots; deep root straight; superficial root pointed; evenly curved shaft, and short point. Ventral anchor outer 37 (35–39; n = 13); inner 32 (27–43; n = 13); base 23 (19–26; n = 13). Dorsal anchor outer 29 (26–32; n = 13); inner 22 (18–25; n = 13); base 19 (16–20; n = 13). Hooks with ancyrocephaline distribution. Hooks similar in shape and size; point and shaft delicate, erect thumb, shank expanded, tapering abruptly proximally in a point, 15 (13–16; n = 70); FH loop about 3/4 shank length.
Remarks
Cosmetocleithrum brachylecis n. sp. resembles C. falsunilatum and C. undulatum n. sp. regarding the shape of the MCO, which is similar to the unique feature of Unilatus. While the MCO in C. falsunilatum has a cork-screw-like shape, the MCO in the new species is formed by a spiral tube with sclerotized walls. The new species differs from C. undulatum n. sp. regarding the lengths of the MCO and accessory piece: in the new species, the accessory piece is straight and elongated, such that it goes beyond the base of the cirrus, while in C. undulatum it is also straight, but only reaches just less than half of the length of the MCO. The new species also differs from all congeneric species in terms of the morphology of the hooks, which are distally slender, expanded in the middle third, and taper abruptly proximally.
Cosmetocleithrum ludovicense n. sp. (Fig. 3)
urn:lsid:zoobank.org:act:2733EAE7-3CF5-4BBD-804A-12852B7348E7
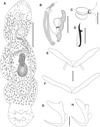 |
Figure 3 Cosmetocleithrum ludovicense n. sp. from Platydoras brachylecis. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Vagina; (D) Hook; (E) Dorsal bar; (F) Ventral bar; (G) Ventral anchor; (H) Dorsal anchor. Scale bars: A, 100 μm, B, C, 20 μm, D–H, 10 μm. |
Type-host: Platydoras brachylecis Piorski, Garavello, Arce H & Sabaj Pérez, 2008 (Siluriformes, Doradidae).
Site in host: Gill lamellae.
Type-locality: Marketplace of the Cidade Operária, São Luís, State of Maranhão, Brazil (2°34′18.0″S 44°11′49.9″W).
Parasitological indexes: Total number of hosts: 3; number of infected hosts: 3; total number of parasites: 434.
Type-material: Holotype CHIOC 40251; Paratypes CHIOC 40252 a–c; 40253 a–q.
Etymology: The specific name is in honor of people born on São Luís Island, state of Maranhão, Brazil.
Description
Based on 25 specimens: 20 mounted in Hoyer’s medium and 5 stained in Gomori’s trichrome. Body fusiform, elongated, comprising cephalic region, trunk, peduncle and haptor. Body, including haptor, 510 (310–630; n = 20) long by 119 (50–170; n = 20) width at level of germarium. Tegument thin, smooth. Cephalic lobes poorly developed. Four bilateral pairs of head organs. Cephalic glands indistinct. Eyes absent. Accessory granules sometimes scattered in the pharyngeal region. Mouth subterminal. Pharynx spherical, weakly muscular 36 (33–50; n = 12) in diameter. Esophagus short. Two intestinal ceca confluent just posteriorly to testis, lacking diverticula. Gonads intercecal, tandem. Germarium 35 and 63; long by 22 and 40 wide. Vagina well-developed, formed by two chambers with heavily sclerotized walls that come together to form an opening; vaginal canal very long, and convoluted, making some loops in the direction of MCO. Seminal receptacle small, slightly rounded. Mehlis’ gland, uterus, oviduct and ootype not observed. Vitellaria extends throughout the trunk, except in areas of other reproductive organs. Testis postgermarial 31 (21–38; n = 4) long by 21 (15–30; n = 4) wide. Vas deferens looping left intestinal cecum; seminal vesicle a dilatation of vas deferens. Prostatic reservoir pyriform. Copulatory complex comprising MCO and accessory piece. MCO a sclerotized tube, inverted J-shaped, 57 (47–67; n = 23) in total length; distal part formed by a small and delicate tube, presenting a cap through which its tip penetrates. MCO base irregularly expanded, with an oval sclerotized brim associated with the base and a conspicuous rod-shape flange. Accessory piece straight and robust, with a hollow structure and sclerotized walls, 40 (33–44; n = 23) long, non-articulated to the base of the MCO. Peduncle short. Haptor, subhexagonal, with dorsal, ventral anchor/bar complex, 94 (45–130; n = 15) wide. Hooks with ancyrocephaline distribution. Anchors similar in shape and size, with well-developed roots. Ventral anchor with elongate and truncated superficial root and short deep root, straight shaft and long point; point passing tip of superficial root, outer 23 (21–25; n = 23); inner 16 (12–20; n = 23); base 16 (10–20; n = 23). Dorsal anchor with elongated and pointed superficial root and short deep root, straight shaft and long point; point passing tip of superficial root, outer 23 (21–25; n = 23); inner 15 (13–18; n = 23); base 15 (13–18; n = 23). Ventral and dorsal bar open V-shaped, with a fracture of the medium region. Ventral bar 48 (20–65; n = 23) wide. Dorsal bar with two submedial projections, 43 (20–59; n = 24) wide. Hooks similar in size and shape, all of them comprising a curved base, slender shank, rounded and protruding thumb, curved shaft and short point 14 (12–15; n = 77) long; FH loop almost the total shank length.
Remarks
Cosmetocleithrum ludovicense n. sp. is closely related to Cosmetocleithrum confusus and to Cosmetocleithrum sobrinus Kritsky, Thatcher & Boeger, 1986 regarding the enlarged base of the MCO. However, it differs from these species in terms of the shape of the accessory piece, which in C. confusus is a hollow structure with sclerotized walls and truncated end, and in C. sobrinus is large, globose and apparently hollow, while in the new species, the MCO is formed by a thin hollow tube of inverted J-shaped, with sclerotized walls. The new species is also closer to Cosmetocleithrum akuanduba, in which the MCO has a tubular coiled shaft that frequently appears to have an inverted J-shaped but differs from the latter in terms of its sclerotized and bulbous base in the latter.
Cosmetocleithrum sacciforme n. sp. (Fig. 4)
urn:lsid:zoobank.org:act:E6C1543F-3AA1-438A-8AF7-12CDE13180F5
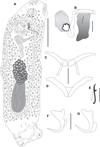 |
Figure 4 Cosmetocleithrum sacciforme n. sp. from Oxydoras niger. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Dorsal bar; (D) Ventral bar; (E) Hook; (F) Ventral anchor; (G) Dorsal anchor. Scale bars: A, 100 μm, B, E, 10 μm, C, D, F, G, 20 μm. |
Type-host: Oxydoras niger (Valenciennes, 1821) (Siluriformes, Doradidae).
Site in host: Gill lamellae.
Type-locality: Juruá River, Acre, Brazil (7°40′34.1″S, 72°39′39.5″W).
Parasitological indexes: Total number of hosts: 7; number of infected hosts: 2; total number of parasites: 7.
Specimens deposited: Holotype CHIOC 40254; Paratypes CHIOC 40255 a–b; 40256 a–d.
Etymology: The specific name is from Latin (saccus = sac; formis = shape of) and refers to the shape of accessory piece, which resembles a sac-like structure.
Description
Based on 7 specimens mounted in Hoyer’s medium. Body fusiform, robust, comprising cephalic region, trunk, peduncle and haptor. Body length, including haptor, 530 (370–660; n = 7) by 184 (70–230; n = 7) width at the level of germarium. Tegument thin, smooth. Cephalic margin broad; cephalic lobes moderately developed; three bilateral pairs of head organs; cephalic glands posterolateral to pharynx. Eyes absent. Accessory granules sometimes scattered in the pharyngeal region. Mouth subterminal. Pharynx spherical, muscular 38 (25–50; n = 4) in diameter. Esophagus short. Two intestinal ceca confluent just posteriorly to testis, lacking diverticula. Gonads intercecal, tandem. Germarium 111 (88–133; n = 5) long by 53 (43–60; n = 5). Vagina not observed; vaginal canal short with aperture sinistroventral. Seminal receptacle rounded. Mehlis’ gland, uterus, oviduct and ootype not observed. Vitellaria extends throughout the trunk, except in areas of other reproductive organs. Testis postgermarial 119 (100–137; n = 5) long by 63 (52–80; n = 5) wide. Vas deferens looping left intestinal cecum; seminal vesicle a dilatation of vas deferens. Prostatic glands forming a dense mass around of the prostatic reservoir. Prostatic reservoir piriform. Copulatory complex comprising MCO and accessory piece. MCO formed by a sclerotized tube slightly flattened and twisted with the same thickness over its entire length 48 (42–55; n = 7) in total length. Accessory piece formed by a single plate of saccular appearance with apparently membranous walls 23 (12–31; n = 7); MCO base with a conspicuous flap, 22 (21–23; n = 3) long. Peduncle very short. Haptor subhexagonal, with dorsal, ventral anchor/bar complex 117 (60–150; n = 7) wide. Hooks with ancyrocephaline distribution. Anchors similar in shape and size, deep and superficial roots well-developed, shaft curved, point straight. Ventral anchor outer 31 (30–32; n = 6); inner 19 (17–21; n = 6); base 18 (17–20; n = 6). Dorsal anchor outer 31 (30–32; n = 6); inner 21 (20–22; n = 6); base 18 (17–19; n = 6). Ventral bar open V-shaped with tapered ends, 60 (54–67; n = 6) wide. Dorsal bar V-shaped, with two submedial long projections directed posteriorly, 65 (45–82; n = 6). Hooks similar in shape and size, with erect thumb, straight shaft, and point; shank bent back proximally 16 (15–17; n = 35). FH loop almost the total shank length.
Remarks
Cosmetocleithrum sacciforme n. sp. differs from all congeneric species mainly in terms of the morphology of the accessory piece, which is formed by a single plate of saccular appearance. The new species is similar to C. bifurcum Mendoza-Franco, Mendoza-Palmero & Scholz, 2016 with regard to the presence of a dense mass of prostatic glands in the anterior trunk but differs in the morphology of the anchors and copulatory complex.
Cosmetocleithrum basicomplexum n. sp (Fig. 5)
urn:lsid:zoobank.org:act:EEAF4C09-5CB9-492B-946A-27D665D790B7
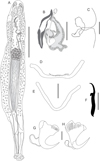 |
Figure 5 Cosmetocleithrum basicomplexum n. sp. from Oxydoras niger. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; C) Vagina; (D) Dorsal bar; (E) Ventral bar; (F) Hook; (G) Dorsal anchor; (H) Ventral anchor. Scale bars: A, 50 μm, B, 40 μm, C, G, H, 20 μm, D, E, 30 μm, F, 10 μm. |
Type-host: Oxydoras niger (Valenciennes, 1821) (Siluriformes, Doradidae).
Site in host: Gill lamellae.
Type-locality: Juruá River, Acre, Brazil (7°40′34.1″S, 72°39′39.5″W).
Parasitological indexes: Total number of hosts: 7; number of infected hosts: 2; total number of parasites: 30.
Specimens deposited: Holotype CHIOC 40249 a; Paratypes CHIOC 40249 b–q; 40250.
Etymology: The specific name is from Latin (basis = base; complexum = complex) and refers to the ornamentation that almost surrounds the base of the MCO.
Description
Based on 23 specimens: 15 mounted in Hoyer’s medium and 8 stained in Gomori’s trichrome. Tegument thin, smooth. Body fusiform, very elongated, comprising cephalic region, trunk, peduncle, and haptor. Body length, including haptor, 2,768 (2,100–3,800; n = 23) by 531 (300–1,350; n = 23) width at the level of germarium. Cephalic lobes poorly developed. Three bilateral pairs of head organs. Cephalic glands posterolateral to pharynx. One pair of eyes. Mouth subterminal. Pharynx subspherical, muscular 175 (135–250; n = 20) by 302 (130–430; n = 20) wide. Esophagus short. Two intestinal ceca confluent just posteriorly to testis, lacking diverticula. Gonads intercecal, testes dorsal to germarium. Germarium 196 (180–235; n = 9) long by 177 (100–185; n = 9). Vagina formed by two chambers; vaginal aperture sinistroventral. Seminal receptacle indistinct. Mehlis’ gland, uterus, oviduct and ootype not observed. Vitellaria extends throughout the trunk, except in areas of other reproductive organs. Testis postgermarial 908 (775–1,025; n = 9) long by 152 (125–205; n = 9) wide. Vas deferens looping left intestinal cecum; seminal vesicle a dilatation of vas deferens. Prostatic reservoir present. Copulatory complex comprising MCO and accessory piece. MCO a sclerotized coiled tube, with wide base opening, 70 (62–82; n = 14) in total length. Straight and robust accessory piece, with an apparently hollow structure and sclerotized walls, serving as a guide for the MCO 76 (62–90; n = 14); MCO base broad and sclerotized edges approximately the length of the MCO 77 (50–60; n = 14) long. Peduncle very long. Haptor subhexagonal, very small in relation to the body, with dorsal, ventral anchor/bar complex 269 (155–490; n = 22) wide. Hooks with ancyrocephaline distribution. Anchors dissimilar in shape, deep and superficial roots well-developed, shaft curved, point straight. Ventral anchor outer 51 (47–55; n = 14) long; inner 25 (20–30; n = 14); base 37 (32–42; n = 14). Dorsal anchor outer 51 (48–55; n = 14) long; inner 25 (20–38; n = 14); base 35 (32–38; n = 14). Ventral bar thin, V-shaped with midline grooves 102 (80–130; n = 14) wide. Dorsal bar thin and fragile W-shaped or arch-shaped, tapering in the medial region from where a small and very fragile projection emerges, often almost imperceptible 108 (87–126; n = 7) wide. Hooks similar in shape and size, thumb short, depressed, shaft strongly robust through its length, tapered abruptly in the final portion of the shaft, forming a small pointed structure 20 (18–21; n = 42). FH loop almost the total shank length.
Remarks
Cosmetocleithrum basicomplexum n. sp is more closely related to C. confusus, C. sacciforme and C. ludovicense regarding the enlarged base of the MCO, but differs from the first two in terms of the morphology of the accessory piece. In the new species, this is straight, with an apparently hollow structure and sclerotized walls opening from half its length, while in C. confusus it is a hollow structure with sclerotized walls and truncated termination and in C. sacciforme it is sac-shaped with apparently membranous walls. The new species closely resembles C. ludovicense n. sp. regarding the morphology of the copulatory complex (i.e. MCO and accessory piece) but differs in terms of the shape of anchors and bars. Cosmetocleithrum basicomplexum n. sp. shares morphological characteristics with C. gigas Morey, Cachique & Babilonia, 2019 regarding the body size and anchor shape but can be differentiated mainly by the morphology of bars. In addition, the hooks of C. basicomplexum n. sp. has a shaft that is strongly robust throughout its length, with a small constricted point, as was observed in the hooks of C. undulatum.
Cosmetocleithrum akuanduba Soares, Neto and Domingues, 2018 (Figs. 6A–6B)
Type-host: Hassar gabiru Birindelli, Fayal & Wosiacki, 2011.
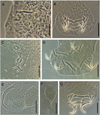 |
Figure 6 (A, B) Cosmetocleithrum akuanduba Soares, Neto and Domingues, 2018: (A) Male copulatory organ; (B) Haptor structures; (C, D) Cosmetocleithrum confusus Kritsky, Thatcher and Boeger, 1986: (C) Male copulatory organ; (D) Haptor structures; (E)–(G) Cosmetocleithrum leandroi Soares, Neto and Domingues, 2018: (E) Male copulatory organ; (F) Egg; (G) Haptor structures. Scale bars: A, 20 μm, B, D, F, G, 40 μm, C, 30 μm, E, F, 50 μm. |
Type-locality: Ilha grande, Xingu River, municipality of Altamira, Pará, Brazil.
Other records: Brazil, Hassar affinis (Steindachner, 1881) Marketplace of the Cidade Operária, São Luís, State of Maranhão (2°34′18.0″S 44°11′49.9″W) (present study); H. gabiru from Iriri River, and Bacajá River, municipality of Altamira, Pará; H. orestis (Steindachner, 1875) from Xingu River, Belo Monte Community, municipality of Vitória do Xingu, Pará [21].
Parasitological indexes: Total number of hosts: 1; number of infected hosts: 1; total number of parasites: 15.
New data
Based on 12 specimens mounted in Hoyer’s medium: Body length, including haptor, 560 (480–650; n = 6) by 110 (90–130; n = 6) width at the level of germarium. Tegument thin, smooth. Eyes and accessory granules absent. Pharynx spherical, muscular 48 (32–65; n = 6) in diameter. Esophagus short. Gonads intercecal, tandem. Testis postgermarial. Copulatory complex comprising MCO and accessory piece. MCO consists of a thin tube forming a semi-ring in a counter-clockwise orientation, J-shaped 94 (79–116; n = 12) in total length. Accessory piece formed by a straight rod, presenting a small gutter in the distal portion serving as a guide for the MCO 31 (21–37; n = 12) long. Haptor subhexagonal, with dorsal, ventral anchor/bar complex, 90 (70–120; n = 9) wide. Anchors similar in shape and size, both of them comprising inconspicuous superficial and deep roots, curved shaft and long and slightly curved point; point acute passing the level of superficial root tip. Ventral anchor with acute superficial root tip 25 (22–29; n = 10) inner; 32 (31–34; n = 10) outer; base 20 (15–24; n = 10) wide; Dorsal anchor with rounded superficial root tip, 26 (23–29; n = 10) inner; 32 (30–35; n = 10) outer; base 19 (14–22; n = 10) wide. Ventral bar slightly arcuate with delicate fracture hatches at medial region and rounded ends 54 (47–63; n = 10) wide. Dorsal bar arcuate, V-shaped, with rounded ends and two submedial long projections directed posteriorly 58 (40–66; n = 10). Hooks with ancyrocephaline distribution, similar in shape and size; non-dilated shank, 14 (13–15; n = 28) long.
Remarks
According to Soares et al. [21], C. akuanduba is characterized mainly by the J-shaped MCO; elongated accessory piece with sharp distal region, distal portion with a small gutter and by the heavily sclerotized vagina with short S-shaped vaginal canal. The specimens studied herein were conformable with the original description, with small differences in the size of the MCO and accessory piece, which in the present study were larger than the specimens described by Soares et al.: [MCO 94 (79–116); accessory piece 31 (21–37) in the present study vs MCO 68 (54–76); accessory piece 23 (18–30)] in Soares et al. [21].
Cosmetocleithrum confusus Kritsky, Thatcher & Boeger, 1986 (Figs. 6C–6D)
Type-host: Oxydoras niger (Valenciennes), Doradidae.
Type-locality: Janauacá Lake near Manaus, Amazonas, Brazil.
Other records: Brazil, Oxydoras niger from Juruá River, State of Acre (7°40′34.1″S, 72°39′39.5″W) (present study); Janauacá Lake, near Manaus, Amazonas State [10]; basin of Solimões River, Amazonas state [20]; Peru, Amazonas River, Iquitos [9].
Parasitological indexes: Total number of hosts: 7; number of infected hosts: 2; total number of parasites: 9.
New data
Based on 11 specimens mounted in Hoyer’s medium. Body fusiform, elongated, comprising cephalic region, trunk, peduncle, and haptor. Body length, including haptor, 883 (620–1040; n = 11) long by 158 (81–185; n = 11) wide, width at the level of germarium. Tegument thin, smooth. Copulatory complex comprising MCO and accessory piece. MCO formed by a coiled sclerotized tube, poorly defined coil, 67 (54–93; n = 11)) in total length, counter-clockwise orientation. Accessory piece 64 (57–75; n = 11) long, non-articulated with MCO, with a proximal portion very tapered and a hollow bulbous portion in the distal region Haptor subhexagonal. Anchors similar in shape; elongate point, short small base; ventral anchor, 31 (31–34; n = 10) long, base 23 (20–35; n = 10); dorsal anchor, 32 (32–39; n = 10), base 24 (22–35; n = 10). Ventral bar V-shaped 85 (72–105; n = 10) long. Dorsal bar V-shaped, with two submedial long projections directed posteriorly 88 (105–87; n = 10) long. Hooks similar in shape and size, tapered shaft and point thumb, straight depressed thumb, slender shank 16 (13–17; n = 40).
Remarks
The morphology of C. confusus from the present study is in agreement with the original description of Kritsky et al. [10], differing only in relation to total body length and bars length, which in the present study were greater than in the specimens described by Kritsky et al.: body length 883 (620–1,040) long by 158 (81–185) wide, ventral bar 85 (72–105) long and dorsal bar 88 (105–87) long, in the present material vs body length 564 (449–706) long by 158 (81–185) wide, dorsal bar 53 (47–62) long and ventral bar 59 (47–74) long in the material of Kritsky et al. [10].
Cosmetocleithrum leandroi Soares, Neto & Domingues, 2018 (Figs. 6E–6F)
Type-host: Hassar gabiru Birindelli, Fayal & Wosiacki, 2011, Doradidae.
Type-locality: Bacajá River, municipality of Altamira, Pará, Brazil.
Others records: Brazil, Hassar affinis (Steindachner, 1881) from Market-place of the Cidade Operária, São Luís, State of Maranhão (2°34′18.0″S 44°11′49.9″W) (present study); H. gabiru from Ilha Grande, Xingu River, and Iriri River, municipality of Altamira, Pará [21].
Parasitological indexes: Total number of host: 1; number of infected hosts: 1; total number of parasites: 93.
New data
Based on 13 specimens mounted in Hoyer’s medium. Body fusiform, elongated, comprising cephalic region, trunk, peduncle, and haptor. Body length, including haptor, 933 (650–1,250; n = 13) long by 119 (110–175; n = 13) wide width at the level of germarium. Tegument thin, smooth. Eyes and accessory granules absent. Pharynx spherical, muscular 62 (50–80; n = 8) in diameter. Vagina heavily sclerotized, vaginal pore sinistroventral; vaginal canal very long, convoluted, looping towards the MCO. Copulatory complex comprising MCO and accessory piece. MCO formed by a coiled sclerotized tube with a bulbous structure in the distal region 612 (546–664; n = 7) in total length, with 3½ to 4½ counter-clockwise rings. Accessory piece 112 (98–138; n = 13) long, not articulated with MCO, comprising a sigmoid rod, with cup-shaped distal region. Peduncle long. Haptor subhexagonal, 109 (110–175; n = 10) wide. Ventral anchors with pointed superficial root and deep root broad, slightly curved shaft and point, outer 47 (44–50; n = 13) long, inner 34 (31–39; n = 13), base 30 (24–33; n = 13); dorsal anchor, with pointed superficial root and deep root short, slightly curved shaft and point, outer 37 (31–43; n = 13), inner 33 (29–36; n = 13), base 23 (20–30; n = 13). Ventral bar straight with knobbed ends 49 (42–55; n = 13) long, by 6 (5–10; n = 13) wide. Dorsal bar straight, with two submedial long projections directed posteriorly 55 (50–65; n = 13) long by 7 (5–8; n = 13). Hooks similar in shape and size, inconspicuous rounded thumb, shaft straight, short, slightly dilated 15 (14–15; n = 40). Egg oval, with filament opposite to opercular end, 84 (80–87; n = 5) long by 50 (47–52; n = 5) wide; filamentous 58 (47–70; n = 5).
Remarks
Cosmetocleithrum leandroi was described from the gill filaments of Hassar gabiru, in the state of Pará, Brazil. According to the original description, C. leandroi is characterized by a MCO comprising a coil of about 3½ rings, a sigmoid accessory piece with a cup-shaped distal portion, a single type of hooks, and anchors with poorly differentiated roots. The specimens studied here, based on newly collected specimens from H. affinis, were in accordance with the original description, while presenting some differences in morphology and measurements, compared with the original material, deposited in CHIOC (Holotype 39053 a, paratypes 39053 b–f, vouchers 39054 a–c): body length 933 (650–1,250) long by 119 (110–175) wide, MCO with 3½ to 4½ counter-clockwise rings, ventral and dorsal anchors with pointed superficial root and deep root developed and hooks with slightly dilated shank, in the present material vs body length 712 (575–835) long by 132 (102–157) wide, MCO with 3½ counter-clockwise rings, superficial and deep roots poorly developed and hooks with non-dilated shank in the original description and type material examined.
Discussion
Cosmetocleithrum is one of the most diverse genera of dactylogyrids parasitizing neotropical catfishes [4]. It is characterized by the presence of two submedial ribbon-like projections on the dorsal bar, a copulatory complex comprising a variably coiled cirrus with counter-clockwise rings and an elaborate accessory piece non-articulated to the cirrus base [10].
So far, species of Cosmetocleithrum have been found in fishes caught in Argentina (1), Brazil (18), and Peru (4). Among these, 14 species parasitize hosts of the Doradidae: C. akuanduba, C. bifurcum, and C. leandroi parasitizing Hassar gabiru; Cosmetocleithrum phryctophallus Soares, Santos Neto & Domingues, 2018 parasitizing Hassar orestis (Steindachner); C. confusus, Cosmetocleithrum gigas, C. gussevi Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum parvum Kritsky, Thatcher & Boeger, 1986, Cosmetocleithrum rarum Kritsky, Thatcher & Boeger, 1986 and Cosmetocleithrum sobrinus Kritsky, Thatcher & Boeger, 1986 parasitizing O. niger; Cosmetocleithrum tortum Mendoza-Franco, Mendoza-Palmero & Scholz, 2016 parasitizing Nemadoras hemipeltis (Eigenmann), Cosmetocleithrum infinitum Morey, Rojas & Cachique, 2022 parasitizing Anadoras grypus (Cope); C. falsunilatum parasitizing Megalodoras uranoscopus (Eigenmann & Eigenmann) and Cosmetocleithrum trachydorasi (Acosta, Scholz, Blasco-Costa, Alves, Silva & 2018) Cohen, Justo, Gen & Boeger, 2020 parasitizing Trachydoras paraguayensis (Eigenmann & Ward).
Cohen et al. [4] proposed two morphologically distinguished groups among the species of Cosmetocleithrum: (1) species that resemble the type species, Cosmetocleithrum gussevi, which present non-articulated bars and a distally bifid accessory piece, often resembling a hook (C. parvum, C. rarum, C. sobrinus, Cosmetocleithrum longivaginatum Suriano & Incorvaia, 1995, C. striatuli, C. laciniatum, C. phryctophallus and C. gigas); and (2) species with articulated bars and a variably shaped accessory piece (e.g. C. confusus, C. bulbocirrus, C. tortum and C. bifurcum). Subsequently, new species of Cosmetocleithrum were proposed and can be placed in those groups. Among these, C. berecae Cohen, Justo, Gen & Boeger, 2020, C. nunani Cohen, Justo, Gen & Boeger, 2020, C. falsunilatum Feronato, Razzolini, Morey & Boeger, 2022, C. galeatum Yamada, Yamada & Silva, 2020, C. spathulatum Yamada, Yamada & Silva, 2020 and C. baculum Yamada, Yamada & Silva, 2020 were placed in group 1. In the description of Cosmetocleithrum infinitum, Morey et al. [13] present images that showed the articulation on the ventral bar. Among the new species proposed in the present study, only C. ludovicense and C. sacciforme present articulated bars and thus are included in group 2, while C. undulatum, C. brachylecis and C. basicomplexum are included in the group 1.
Nevertheless, in the phylogenetic analysis of Cosmetocleithrum conducted by Mendoza-Palmero et al. [11], the phylogenetic position of the species analyzed corresponded partially to the morphological categories proposed by Cohen et al. [4]. Their study [11] provides suggestions for further studies regarding Cosmetocleithrum spp., including the molecular characterization of the remaining species of this genus in order to evaluate the phylogenetic positions of all the species.
Mendoza-Palmero et al. [11] stated that more than 15 species of Cosmetocleithrum have been described over recent years, thereby adding new morphological characteristics that can be included in the diagnosis of the genus. They cited C. bifurcum (a member of the “doradid group”) and C. baculum (a member of the “auchenipterid group”), which have dissimilar hooks, but C. nunani (also a member of the auchenipterid group) presents two morphologically distinct hooks. Regarding the species described in the present paper, C. undulatum (a member of the doradid group) can be included in this set of species with two morphological distinct hooks, thus showing that it is not related to the host family. Considering the position of the vagina, a feature also mentioned by Mendoza-Palmero et al. [11], the species described herein are concordant with almost all species of the genus, presenting a sinistral aperture, with the exception of C. tortum.
Despite the high diversity of catfish in the Amazon region and the economic importance of some of these species, knowledge of the helminth fauna parasitizing these fish is still fragmentary and far from sufficient [12]. Moreover, no studies on the helminth fauna of many of these fish have yet been conducted, as is the case of P. brachylecis, which is a thorny catfish endemic to the basins of the Mearim River, Pindaré River, Itapecuru River and Parnaíba River, in northeastern Brazil [16]. The new species described in the present study and the new records of hosts for P. brachylecis demonstrate that there is a real need to expand such studies, especially with regard to endemic fish species and those that have recently been described. The finding, more than 30 years later, of two new species in O. niger, the type host of the first four species that were proposed for Cosmetocleithrum, also demonstrates that studies on these hosts are necessary and should take into consideration the ecological processes related to the host-parasite association.
Acknowledgments
This work was supported by “Coordenação de Aperfeiçoamento Técnico de Pessoal de Nível Superior/CAPES” (finance cod 001, Process: 88887.707472/2022-00. “Programa CAPES: PDPG Emergencial de Consolidação Estratégica dos Programas de Pós-Graduação (PPGs)” Stricto sensu academics with grades 3 and 4 and “Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão” (grant number UNIVERSAL-01148/18-, grant number BD-01172/20).
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Abdallah VD, Azevedo RK, Luque JL. 2012. Three new species of Monogenea (Platyhelminthes) parasites of fish in the Guandu River, southeastern Brazil. Acta Scientiarium Animal Science, 34, 483–490. [Google Scholar]
- Albert JS, Reis RE. 2011. Introduction to Neotropical freshwater fish, in Historical Biogeography of Neotropical Freshwater Fishes. Albert JS, Reis RE, Editors. University of California Press: California. p. 3–19. [Google Scholar]
- Cassemiro FAS, Albert JS, Antonelli A, Menegotto A, Wüest RO, Cerezer F, Coelho MTP, Reis RE, Tan M, Tagliacollo V, Bailly D, da Silva VFB, Frota A, da Graça WJ, Ré R, Ramos T, Oliveira AG, Dias MS, Colwell RK, Rangel TF, Graham CH. 2023. Landscape dynamics and diversification of the megadiverse South American freshwater fish fauna. Proceedings of the National Academy of Sciences, 120, e2211974120. [CrossRef] [PubMed] [Google Scholar]
- Cohen SC, Justo MCN, Gen DC, Boeger WA. 2020. Dactylogyridae (Monogenoidea, Polyonchoinea) from the gills of Auchenipterus nuchalis (Siluriformes, Auchenipteridae) from the Tocantins River. Parasite, 27, 4. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Feronato SG, Razzolini E, Morey GAM, Boeger WA. 2022. Neotropical Monogenoidea 64. Cosmetocleithrum falsunilatum sp. n. (Monogenoidea, Dactylogyridae) parasite of the gills of Megalodoras uranoscopus (Siluriformes, Doradidae) from the Solimões River, near Iquitos, Peru. Systematic Parasitology, 99, 41–346. [Google Scholar]
- Fricke R, Eschmeyer WN, Van der laan R. 2023. Eschmeyer’s catalog of fishes: Genera, Species, References. Accessed from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp on 2023–19–05. [Google Scholar]
- Graça RJ, Ueda BH, Oda FH, Takemoto RM. 2013. Monogenea (Platyhelminthes) gill parasites of Hoplias malabaricus (Bloch, 1794) (Pisces: Erythrinidae) in the Várzea of the Upper Paraná River, Paraná and Mato Grosso do Sul States, Brazil. Checklist, 9, 1484–1487. [CrossRef] [Google Scholar]
- Humason GL. 1979. Animal tissue techniques. W.H. Freeman Co.: USA. p. 661. [Google Scholar]
- Iannacone JA, Luque JL. 1991. Monogeneos parasitos del “paiche” Arapaima gigas (C.) y del “turushuqui” Oxydoras niger (V.) en la Amazonia peruana. Boletin de Lima, 76, 43–48. [Google Scholar]
- Kritsky DC, Thatcher VE, Boeger WA. 1986. Neotropical Monogenea. 8. Revision of Urocleidoides (Dactylogyridae, Ancyrocephalinae). Proceedings of the Helminthological Society of Washington, 53, 1–37. [Google Scholar]
- Mendoza-Palmero C, Acosta A, Scholz T. 2022. Molecular phylogeny of Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986 (Monogenoidea: Dactylogyridae), gill parasites of Neotropical catfishes (Siluriformes). Journal of Helminthology, 96, E56. [CrossRef] [PubMed] [Google Scholar]
- Mendoza-Palmero CA, Scholz T, Mendoza-Franco EF, Kuchta R. 2012. New species and geographical records of dactylogyrids (Monogenea) of Catfish (Siluriformes) from the Peruvian Amazonia. Journal of Parasitology, 98, 484–497. [CrossRef] [PubMed] [Google Scholar]
- Morey GAM, Rojas CAT, Cachique JCZ. 2022. Cosmetocleithrum infinitum sp. n. (Monogenoidea: Dactylogyridae) parasite of the gills of Anadoras grypus (Siluriformes: Doradidae) from the Amazonas River, in Loreto, Peru. Systematic Parasitology, 99, 683–688. [CrossRef] [PubMed] [Google Scholar]
- Pelicice FM, Azevedo-Santos VM, Vitule JRS, Orsi ML, Lima Junior DP, Magalhães ALB, Pompeo PS, Petrere JM, Augustine AA. 2017. Neotropical freshwater fishes imperiled by unsustainable policies. Fish and Fisheries, 18, 1119–1133. [CrossRef] [Google Scholar]
- Perera ES, Mauad JRC, Takemoto RM, Lima-Junior SE. 2018. Fish parasite diversity in the Amambaí River, State Mato Grosso do Sul, Brazil. Acta Scientiarum Biological Sciences, 40, e36330. [CrossRef] [Google Scholar]
- Piorski NM, Garavello JC, Arce Mariangeles, Sabaj Pérez MH. 2008. Platydoras brachylecis, a new species of thorny catfish (Siluriformes: Doradidae) from northeastern Brazil. Neotropical Ichthyology, 6, 481–494. [CrossRef] [Google Scholar]
- Rasband W. Image J documentation. Available from: http://rsb.info.nih.gov/ij/docs/index.html Accessed: 09 nov. 2018. [Google Scholar]
- Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. 2016. Fish biodiversity and conservation in South America. Journal of Fish Biology, 89, 12–47. [CrossRef] [PubMed] [Google Scholar]
- Sabaj MH, Arce HM. 2021. Towards a complete classification of the Neotropical thorny catfishes (Siluriformes: Doradidae). Neotropical Ichthyology, 19, e210064. [CrossRef] [Google Scholar]
- Silva AMO, Tavares-Dias M, Jeronimo GT, Martins ML. 2011. Parasite diversity in Oxydoras niger (Osteichthyes: Doradidae) from the basin of Solimões River, Amazonas State, Brazil, and the relationship between monogenoidean and condition factor. Brazilian Journal of Biology, 71, 791–796. [CrossRef] [PubMed] [Google Scholar]
- Soares GB, Neto JFS, Domingues MV. 2018. Dactylogyrids (Platyhelminthes: Monogenoidea) from the gills of Hassar gabiru and Hassar orestis (Siluriformes: Doradidae) from the Xingu Basin, Brazil. Zoologia, 35, e23917. [CrossRef] [Google Scholar]
- Suriano DM, Incorvaia IS. 1995. Ancyrocephalid (Monogenea) parasites from siluriform fishes from the Paranean Platean icthyogeographical province in Argentine. Acta Parasitologica, 40, 113–124. [Google Scholar]
- Yamada POF, Yamada FH, Silva RJ, Anjos LA. 2017. A new species of Cosmetocleithrum (Monogenea, Dactylogyridae), a gill parasite of Trachelyopterus galeatus (Siluriformes, Auchenipteridae) from Brazil, with notes on the morphology of Cosmetocleithrum striatuli. Comparative Parasitology, 84, 119–123. [Google Scholar]
- Yamada POF, Yamada FH, Silva RJ. 2020. Three new species of Cosmetocleithrum (Monogenea: Dactylogyridae) gill parasites of Trachelyopterus galeatus (Siluriformes: Auchenipteridae) in Southeastern Brazil. Acta Parasitologica, 66, 436–445. [Google Scholar]
Cite this article as: Silva ALS, Meneses YC, Martins WMO, Cohen SC, Costa AP & Justo MCN. 2023. Dactylogyrids (Platyhelminthes, Monogenea) from the gill lamellae of doradids (Siluriformes) with description of five new species of Cosmetocleithrum and new geographical distribution for known species from the Neotropical Region, Brazil. Parasite 30, 53.
All Figures
 |
Figure 1 Cosmetocleithrum undulatum n. sp. from Platydoras brachylecis. (A) Total ventral view (composite); (B) Copulatory complex, ventral view; (C) Hook pairs 1–4, 6, 7; (D) Hook pair 5; (E) Dorsal bar; (F) Ventral bar; (G) Ventral anchor; (H) Dorsal anchor. Scale bars: A, 100 μm, B, C, 10 μm, D, 50 μm, E, F, 15 μm, G, H, 20 μm. |
| In the text | |
 |
Figure 2 Cosmetocleithrum brachylecis n. sp. from Platydoras brachylecis. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Hook; (D) Dorsal bar; (E) Ventral bar; (F) Ventral anchor; (G) Dorsal anchor. Scale bars: A, 100 μm, B, D–G, 20 μm, C, 10 μm. |
| In the text | |
 |
Figure 3 Cosmetocleithrum ludovicense n. sp. from Platydoras brachylecis. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Vagina; (D) Hook; (E) Dorsal bar; (F) Ventral bar; (G) Ventral anchor; (H) Dorsal anchor. Scale bars: A, 100 μm, B, C, 20 μm, D–H, 10 μm. |
| In the text | |
 |
Figure 4 Cosmetocleithrum sacciforme n. sp. from Oxydoras niger. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; (C) Dorsal bar; (D) Ventral bar; (E) Hook; (F) Ventral anchor; (G) Dorsal anchor. Scale bars: A, 100 μm, B, E, 10 μm, C, D, F, G, 20 μm. |
| In the text | |
 |
Figure 5 Cosmetocleithrum basicomplexum n. sp. from Oxydoras niger. (A) Total, ventral view (composite); (B) Copulatory complex, ventral view; C) Vagina; (D) Dorsal bar; (E) Ventral bar; (F) Hook; (G) Dorsal anchor; (H) Ventral anchor. Scale bars: A, 50 μm, B, 40 μm, C, G, H, 20 μm, D, E, 30 μm, F, 10 μm. |
| In the text | |
 |
Figure 6 (A, B) Cosmetocleithrum akuanduba Soares, Neto and Domingues, 2018: (A) Male copulatory organ; (B) Haptor structures; (C, D) Cosmetocleithrum confusus Kritsky, Thatcher and Boeger, 1986: (C) Male copulatory organ; (D) Haptor structures; (E)–(G) Cosmetocleithrum leandroi Soares, Neto and Domingues, 2018: (E) Male copulatory organ; (F) Egg; (G) Haptor structures. Scale bars: A, 20 μm, B, D, F, G, 40 μm, C, 30 μm, E, F, 50 μm. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


