Figure S1:
Download original image
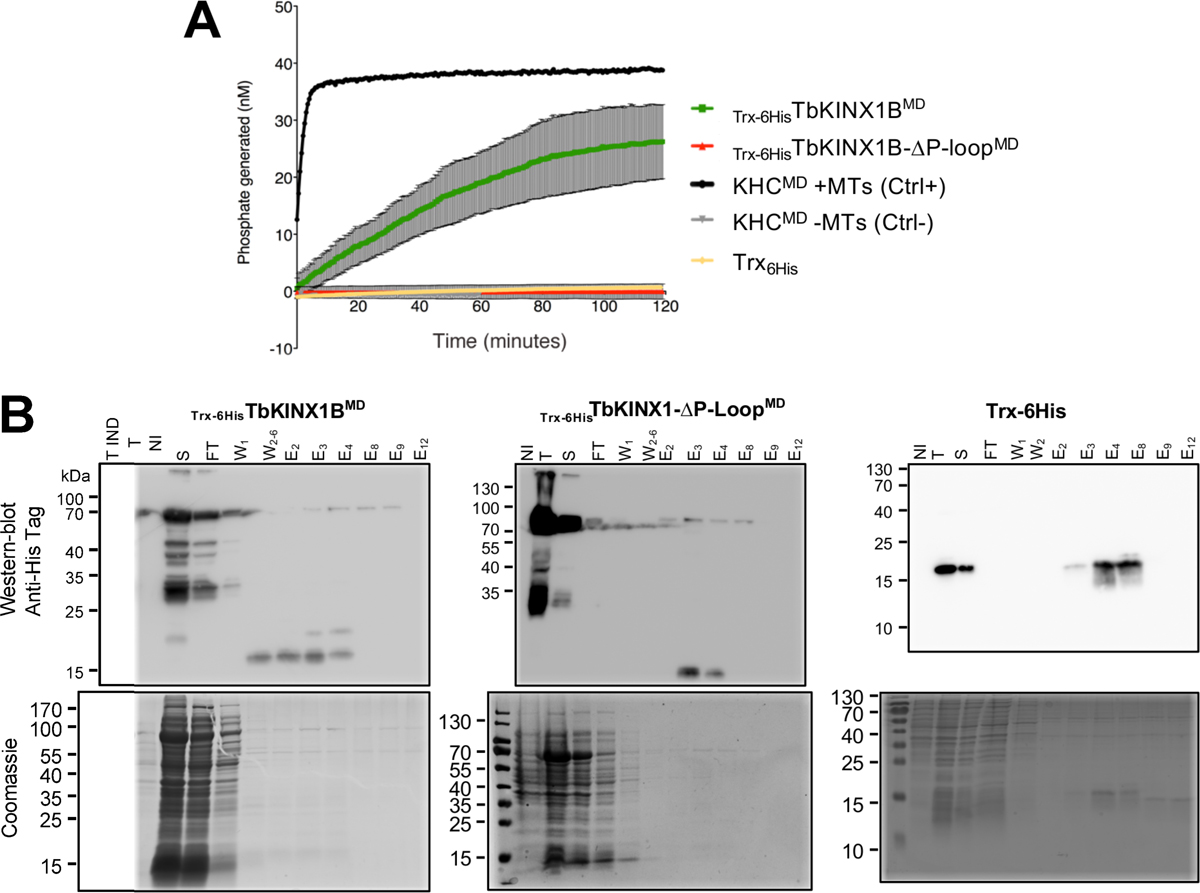
TbKINX1B can hydrolyse ATP in vitro. A. ATPase activity using the ELIPA in vitro assay. Kinesin activity was measured by the amount of generated phosphate per minute, in the presence of taxol-stabilized microtubules and ATP. The human kinesin heavy chain motor domain (KHCMD) is the positive control kinesin in presence of MTs (+MTs, black line) or negative control in the absence of MT (−MTs, grey line), the TbKINX1BMD (green), TbKINX1BMD deleted for the ATPase domain (ΔP-loopMD, red line), and 6HisTRX purified protein (yellow line), (n = 3). B. Recombinant TbKINX1B proteins purification. Western blot analysis (upper panel) and Coomassie-stained SDS-PAGE (lower panel) of the purification of the Trx-6HisTbKINX1BMD (A), Trx-6HisTbKINX1B-ΔP-loopMD (B), and Trx-6His (C) proteins. The recombinant proteins were immunolabelled using anti-Histidine tag. Abbreviations: Total (T), non-induced (NI), induced (IND), supernatant (S), flow-through (FT), wash (W, different wash fractions numbered), Elution (E, fractions numbered).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


