| Issue |
Parasite
Volume 23, 2016
|
|
|---|---|---|
| Article Number | 7 | |
| Number of page(s) | 10 | |
| DOI | https://doi.org/10.1051/parasite/2016007 | |
| Published online | 22 February 2016 | |
urn:lsid:zoobank.org:pub:EDC87FF9-2C01-42E4-A1B7-A5F0377A0DDF
Research Article
Chewing lice of genus Ricinus (Phthiraptera, Ricinidae) deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia, with description of a new species
Mallophages du genre Ricinus (Phthiraptera, Ricinidae) déposés à l’Institut de zoologie de l’Académie des Sciences de Russie, Saint-Pétersbourg, Russie, avec description d’une nouvelle espèce
Department of Biology and Wildlife Diseases, Faculty of Veterinary Hygiene and Ecology, University of Veterinary and Pharmaceutical Sciences, Palackeho tr. 1/3, 612 42
Brno, Czech Republic
* Corresponding author: miroslav.valan@yahoo.com
Received:
6
August
2015
Accepted:
31
January
2016
We revised a collection of chewing lice deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia. We studied 60 slides with 107 specimens of 10 species of the genus Ricinus (De Geer, 1778). The collection includes lectotype specimens of Ricinus ivanovi Blagoveshtchensky, 1951 and of Ricinus tugarinovi Blagoveshtchensky, 1951. We registered Ricinus elongatus Olfers, 1816 ex Turdus ruficollis, R. ivanovi ex Leucosticte tephrocotis and Ricinus serratus (Durrant, 1906) ex Calandrella acutirostris and Calandrella cheleensis which were not included in Price’s world checklist. New records for Russia are R. elongatus ex Turdus ruficollis; Ricinus fringillae De Geer, 1778 ex Emberiza aureola, Emberiza leucocephalos, Emberiza rustica, Passer montanus and Prunella modularis; Ricinus rubeculae De Geer, 1778 ex Erithacus rubecula and Luscinia svecica; Ricinus serratus (Durrant, 1906) ex Alauda arvensis. New records for Kyrgyzstan are R. fringillae ex E. leucocephalos and ex Fringilla coelebs. A new record for Tajikistan is R. serratus ex Calandrella acutirostris. The new species Ricinus vaderi Valan n. sp. is described with Calandra lark, Melanocorypha calandra; from Azerbaijan, as a type host.
Résumé
Nous avons révisé une collection de mallophages déposée à l’Institut de zoologie de l’Académie des Sciences de Russie, Saint-Pétersbourg, Russie. Nous avons examiné 60 lames avec 107 spécimens de dix espèces du genre Ricinus (De Geer, 1778). La collection contient des spécimens lectotypes de Ricinus ivanovi Blagoveshtchensky, 1951 et de Ricinus tugarinovi Blagoveshtchensky, 1951. Nous avons enregistré Ricinus elongatus Olfers, 1816 ex Turdus ruficollis, R. ivanovi ex Leucosticte tephrocotis et Ricinus serratus (Durrant, 1906) ex Calandrella acutirostris et Calandrella cheleensis qui avaient été omis dans la liste mondiale de Price. Les nouvelles mentions pour la Russie sont R. elongatus ex Turdus ruficollis ; Ricinus fringillae De Geer, 1778 ex Emberiza aureola, Emberiza leucocephalos, Emberiza rustica, Passer montanus et Prunella modularis ; Ricinus rubeculae De Geer, 1778 ex Erithacus rubecula et Luscinia svecica; Ricinus serratus (Durrant, 1906) ex Alauda arvensis. Une nouvelle mention pour le Tadjikistan est R. serratus ex Calandrella acutirostris. La nouvelle espèce Ricinus vaderi Valan n. sp. est décrite avec l’alouette calandre Melanocorypha calandra, d’Azerbaidjan, comme hôte-type.
Key words: Ricinus vaderi / New species / Melanocorypha calandra / Phthiraptera / Chewing lice / Blagoveshtchensky’s collection
Miroslav Valan – urn:lsid:zoobank.org:author:1DBA4E7F-9CF1-464B-BCF4-A7D7CFE26E00
Oldrich Sychra – urn:lsid:zoobank.org:author:D28CEAFB-0F34-4937-A66E-6AC8BA90E325
Ivan Literak – urn:lsid:zoobank.org:author:1A328AC3-67A8-4F79-86DB-81E3DB0661C8
© M. Valan et al., published by EDP Sciences, 2016
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Ricinus De Geer, 1778 (Phthiraptera: Amblycera) is the largest genus of chewing lice found parasitizing Passeriformes [17]. Whereas chewing lice mainly feed on feathers, these lice also feed on blood and have an unbalanced sex ratio with approximately 1 male to 10 females [17]. The distribution of many chewing lice is characterized by high host specificity and wide host distribution is therefore relatively rare [19]. Hopkins [10] noted that Ricinus has an anomalous distribution and occurs on approximately one-third of the 70 families of Passeriformes. Major revisions of this genus were done separately for Old World [20] and New World species [17] and comprise the majority of known species.
Price et al. [19] listed 65 species of the genus Ricinus parasitizing 271 different hosts in 32 families and created 299 host-louse associations. In the years thereafter, Ricinus facetus Mey, 2007, Ricinus gutheili Mey, 2007, Ricinus nhillensis Mey, 2007, Ricinus ornatulus Mey, 2007, Ricinus ptilotulae Mey, 2007 [16] and Ricinus ruficapillus Oniki, Mey, Willis, 2004 [18] were described. New host-louse associations have been reported for Ricinus arcuatus (Kellogg and Mann, 1912), Ricinus diffusus (Kellogg, 1896), Ricinus fringillae De Geer, 1778, Ricinus invadens (Kellogg, 1899), Ricinus marginatus (Children, 1836), Ricinus meinertzhageni Rheinwald, 1968, Ricinus mugimaki (Uchida, 1915) and Ricinus pessimalis Eichler, 1956, [7, 9, 11, 22–25] and these bring the total number of species to 71 and host-louse associations to 315.
Following host nomenclature according to Clements et al. [5], lice of the genus Ricinus infest more than one-third of the families of Passeriformes (45 of 122), still in accordance with Hopkins [10] statement. For example, R. fringillae De Geer, 1778 is known from 48 bird species from 9 families [11, 19]. There is no doubt that descriptions of new species and new host-louse records are expected. Consequently, examining museum collections and revision of material deposited worldwide are necessary to obtain more data concerning geographical distribution, biodiversity and host associations of chewing lice. Furthermore, for some of the species, Blagoveshtchensky reported more specimens than we have found in his collection. We hope that with our article, these lost samples will be found in the future.
Blagoveshtchensky [3] described Ricinus ivanovi Blagoveshtchensky, 1951 and Ricinus tugarinovi Blagoveshtchensky, 1951; he also mentioned additional records of this genus in the former USSR. Although he noted that Calandrella acutirostris acutirostris Hume, 1873 (Passeriformes: Alaudidae) was a host of Ricinus serratus (Durrant, 1906), this was not cited by Price et al. [19]. Price et al. [19] included only reliable sources, but because R. serratus is already known from several alaudid hosts, this prompted us to inspect material acquired by Blagoveshtchensky. These specimens are deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg (ZISP) and are part of a larger collection, mainly assembled by him during the 1930s through the 1970s.
In this article, we present data obtained from the studies of Ricinus spp. deposited at the ZISP. We include new country records for Azerbaijan, Kyrgyzstan, Russia and Tajikistan. Knowing that data about the biodiversity of chewing lice within the former USSR were published mainly in Russian and that the existing literature is scarcely accessible, we will refer only to those records we have been able to verify. Accordingly, species of Ricinus recorded so far in Azerbaijan are Ricinus frenatus Burmeister, 1838 ex Regulus regulus buturlini and R. fringillae ex Emberiza schoeniclus [2]. Records in Russia are Ricinus elongatus (Olfers, 1816) ex Turdus pilaris L., 1758 and Turdus merula L., 1758; R. fringillae ex Fringilla coelebs L., 1758 and Ricinus thoracicus Packard, 1870 ex Plectrophenax nivalis (L., 1758) [4, 14]. Records from Tajikistan are Ricinus frenatus (Burmeister, 1838) ex Regulus regulus (L., 1758); R. fringillae ex Fringilla coelebs, Emberiza citrinella L., 1758, Emberiza cia L., 1758 and Prunella collaris (Scopoli, 1769); R. ivanovi ex Leucosticte brandti Bonaparte, 1850; R. serratus ex C. a. acutirostris and Galerida cristata (L., 1758); R. tugarinovi ex Terpsiphone paradisi (L., 1758) [3].
Materials and methods
Chewing lice of the genus Ricinus deposited at the ZISP were examined and described. In this article, we present all Ricinus species found in this collection. Host systematics follow Clements et al. [5]. To each louse species, we added notes as follows: number of females, males, nymphs, host (Order: Family) including common English name, country, location, number of specimens (slide number), date, collector (coll.) and identifier (det.). Slide numbers are equal to accession numbers, but only handwritten catalogues exist. For those slides without slide numbers, we allocated a new slide number in the form MVXY. Notes from slides about locality are rewritten from slide labels while transliterating from Cyrillic to the Latin alphabet without further changes, and we cannot guarantee their validity. Where information may be lacking, this should be considered as a deficiency of information noted by the collector or collection manager. The majority of the examined specimens are in poor condition, mainly due to mounting directly to medium without using any clearing agent prior to mounting. The medium is most likely Canada balsam, but there are no written notes about it. If the case happened to be otherwise, this will be noted below.
Species concept, morphological characters and system of chaetotaxy follow Nelson [17] (see Fig. 2). All measurements are in millimeters and were taken using QuickPhoto Micro 3.0. In order to achieve high quality, drawings were made as vectors using Adobe Illustrator C6.
The newly described chewing louse species is attributed to the first author.
Results and discussion
In total, we examined 107 specimens of the genus Ricinus mounted on 60 slides and no samples in fixatives were found. The majority of these specimens (78) are females, with only 7 males and 22 nymphs. Specimens had been collected from various locations and by several collectors. Specimens from Tajikistan had mostly been examined and data presented by Blagoveshtchensky [3]. Studies of lice obtained in Azerbaijan have also been published [2], with the exception of Ricinus sp. from Melanocorypha calandra. As expected, the sex ratio is unbalanced (1–11.14) and our results correspond with Nelson [17] and literature cited therein, thus showing that females of Ricinus are approximately 10 times more abundant than males.
Phthiraptera Haeckel, 1896
Amblycera Kellogg, 1896
Ricinidae Neuman, 1890
Ricinus De Geer, 1778
Ricinus dolichocephalus (Scopoli, 1763)
Pediculus dolichocephalus Scopoli, 1763: 382.
Type host: Oriolus oriolus (L., 1758) – Eurasian Golden Oriole (Oriolidae).
Type locality: NE Poland.
Material examined: 1♀ (slide no. 158) ex O. oriolus (Passeriformes: Oriolidae), Eurasian Golden Oriole. Russia, Crimea.
Remarks: According to literature to which we had access, this is the first record of R. dolichocephalus in Russia.
Ricinus elongatus (Olfers, 1816)
Nirmus elongatus Olfers, 1816: 88.
Type host: Turdus viscivorus (L., 1758) – Mistle Thrush (Turdidae).
Type locality: Hodonín, Czech Republic.
Material examined: 6♀, 1♂, 1N.
1♀ (slide no. MV01) ex Turdus ruficollis Pallas, 1776 (Passeriformes: Turdidae), Red-throated Thrush. Russia. Stanoviy khrebet: 9.V.1944.
5♀, 1♂, 1N ex Turdus sp. (Passeriformes: Turdidae), Russia. Okr. Turukhanska: 3♀, 1♂, 1N (slide no. MV02), 30.V.1902, Ostrovskikh P. coll.; Tomskaya gub. Elizavetinsk. zav.: 1♀ (slide no. MV03), 5.VII.1903; no specific location: 1♀ (slide no. MV04), 1907, Haritonov coll.
Remarks: Grube [8] reported Physostomum mystax (junior synonym of R. elongatus) on T. ruficollis. Balát [1] in his study, cited Grube [8] and included T. ruficollis as a host of R. elongatus without examining any material. In revision of Ricinus occurring in the Old World [20], Rheinwald did not report any notes about this association and this is probably why it was not cited by Price et al. [19]. We have unfortunately not been able to check Grube’s notes and assume that specimens are not available for study. Having previous unreliable reports and only one specimen in this collection creates an aggravating circumstance. However, R. elongatus is known as a parasite of 11 species of birds from four closely related families, and eight of these are from the genus Turdus (Turdidae). Bearing this in mind, we suggest that T. ruficollis be recognized as a valid host for R. elongatus.
Ricinus frenatus (Burmeister, 1838)
Physostomum frenatum Burmeister, 1838: 442.
Type locality: Not recorded (probably Germany).
Type host: Regulus regulus (L., 1758) – Goldcrest (Regulidae).
Material examined: 10♀.
5♀, ex R. regulus (Passeriformes: Regulidae), Goldcrest. Tajikistan. Zap. Tigrovaya Balka: 1♀ (slide no. 134), 21.XII.1939; 1♀ (slide no. 135), 3.I.1940; 3♀ (slide no. 136), 10.I.1947, Ivanov A. coll., det. Blagoveshtchensky.
5♀ ex Regulus r. buturlini Loudon, 1911 (Passeriformes: Regulidae), Goldcrest (Caucasian). Azerbaijan. Lenkoranskiy r-n d. Alekseevka: 3♀, 10–14.III.1934 (slides no. 130–132), Shtrom Zh. coll., det. Blagoveshtchensky; Sari Island (Caspian Sea): 2♀ (slide no. 133), 14.III.1937, det. Blagoveshtchensky.
Remarks: Specimens were examined and notes were given by Blagoveshtchensky [2, 3].
Ricinus fringillae De Geer, 1778
Ricinus fringillae De Geer, 1778: 71.
Type host: Emberiza citrinella L., 1758 – Yellowhammer (Emberizidae).
Type locality: Saxony, Germany.
Material examined: 30♀, 3♂, 14N.
1♀, 4N (slide no. 2410) ex Carduelis flammea (L., 1758) (Passeriformes: Fringillidae), Common Redpoll. 17.I.1929.
1♀, 7N (slide no. MV05) ex Emberiza aureola Pallas, 1773 (Passeriformes: Emberizidae), Yellow-breasted Bunting. Russia. Okr. n-Tambovska na Amure: 4.VIII.1939, Blagoveshtchensky coll.
2♀, 1♂ (slide No. 156) ex E. citrinella (Passeriformes: Emberizidae), Yellowhammer. Tajikistan. Dushanbe: 15.II.1947, Ivanov A. coll., det. Blagoveshtchensky.
1N (slide no. MV06) ex Emberiza leucocephalos Gmelin, 1771 (Passeriformes: Emberizidae), Pine Bunting. Kyrgyzstan. Naryn: 9.III.1913.
2♀ (slide no. MV07) ex Emberiza rustica Pallas, 1776 (Passeriformes: Emberizidae), Rustic Bunting. Russia. Ural Basseg: 14.VII.1948.
1♀ (slide no. 155) ex Emberiza schoeniclus (L., 1758) (Passeriformes: Emberizidae), Reed Bunting. Azerbaijan. Kumbashi: 28.II.1934, Shtrom Zh. coll., det. Blagoveshtchensky.
15♀, 1♂, 2N, ex Fringilla coelebs L., 1758 (Passeriformes: Fringillidae), Common Chaffinch.
Russia. Petrodvorec: 3♀ (slides no. MV08–10), VI.1952, Karakasheva coll; Tver’ okr.: 1N (slide no. 139), 18.IV.1907, det. Blagoveshtchensky; and 1♀, 1♂ (slide no. 137), 13.IX.1907, Plotnikov V. coll., det. Blagoveshtchensky.
Kyrgyzstan. Naryn: 1♀ (slide no. MV011), the most likely III.1913.
Tajikistan. k. Tazhni: 3♀ (slide no. 138), 25.II.1947, Ivanov A. coll., det. Blagoveshtchensky.
No locality notes: 1♀ (slide no. MV12).
Locality: 6♀, 1N (slide no. 2575) ex F. coelebs (Passeriformes: Fringillidae), Common Chaffinch. Tsikhis-Dzhivari: 1935.
3♀ (slide no. MV13) ex Passer montanus (L., 1758) (Passeriformes: Passeridae), Eurasian Tree Sparrow. Russia. Vologda: 1947.
1♀, 1♂ (slide no. 154) ex Prunella collaris (Scopoli, 1769) (Passeriformes: Prunellidae), Alpine Accentor. Tajikistan. Gissarskiy khrebet pereval Anzob: VIII.1945, Ivanov A. coll., det. Blagoveshtchensky.
3♀ (slide no. MV14) ex Prunella modularis (L., 1758) (Passeriformes: Prunellidae), Dunnock. Russia. Kavkaz: 1917, Satunin coll.
1♀ (slide no. MV15) ex Turdus sp. (Passeriformes: Turdidae). Ukraine. Kiev: 3.IV.1902.
Remarks: According to literature to which we had access, new host records for R. fringillae in Russia are Emberiza aureola, E. leucocephalos, E. rustica, P. montanus and P. modularis.
Ricinus ivanovi Blagoveshtchensky, 1951 (Fig. 1A)
Ricinus ivanovi Blagoveshtchensky, 1951: 283.
 |
Figure 1. (A) Lectotype female of Ricinus ivanovi Blagoveshtchensky, 1951. (B) Lectotype female of R. tugarinovi Blagoveshtchensky, 1951. (C) Holotype female of R. vaderi n. sp. Scale bar: 1 mm. |
Type host: Leucosticte brandti Bonaparte, 1851 – Black-headed Mountain-Finch (Fringillidae).
Type locality: Vewrk, Gissar’s Range, Pamir, Tajikistan.
Material examined: 1♀ (slide no. 152) ex L. brandti (Passeriformes: Fringillidae), Black-headed Mountain-Finch. Tajikistan. Gissarskiy khrebet pereval Anzob: 19.VIII.1945, Ivanov A. coll., det. Blagoveshtchensky.
Remarks: Blagoveshtchensky [3] based his description of R. ivanovi on 1♀. Unfortunately, Blagoveshtchensky did not provide a slide number. Neither Rheinwald [20] nor Nelson [17] examined the type specimen. The specimen examined in this study has the same notes about locality and date as given in the original description. Accordingly, this specimen is designated a lectotype specimen of R. ivanovi. Nelson [17] examined 8♀ and 3♂ of R. ivanovi ex Leucosticte tephrocotis (Swainson, 1832) and none from L. brandti. Nelson’s record was included by Emerson [6] in a chewing lice checklist of North America. Nevertheless, this was probably missed accidentally by Price et al. [19].
Ricinus rubeculae (Schrank, 1776)
Pediculus rubeculae Schrank, 1776: 115.
Type host: Erithacus rubecula (L., 1758) – European Robin (Muscicapidae).
Type locality: Moravia, Czech Republic.
Material examined: 3♀.
2♀ (slides no. MV16–17) ex E. rubecula (Passeriformes: Muscicapidae), European Robin. Russia. Teberda: 13.VI.1917, Gorbunov G. coll.
1♀ (slide no. MV18) ex Luscinia svecica (L., 1758) (Passeriformes: Muscicapidae), Bluethroat. Russia. Kazalinsk: 15.VI.1932, Popov coll.
Remarks: According to literature to which we had access, this is the first record of R. rubeculae in Russia.
Ricinus serratus (Durrant, 1906)
Physostomum serratum Durrant, 1906: 528.
Type host: Eremophila alpestris (L., 1758) – Horned or Przewalski’s Lark (Alaudidae).
Type locality: Ft. Collins, Colorado, USA.
Material examined: 18♀, 3♂, 4N.
1♀ (slide no. MV19) ex Alauda arvensis (L., 1758) (Passeriformes: Alaudidae), Sky Lark. Russia. Verhojanskij okrug: 2.VI.1927, Tkachenko coll.
2♀ (slide no. 151) ex Calandrella acutirostris acutirostris Hume, 1873 (Passeriformes: Alaudidae), Hume’s Lark. Tajikistan. Dushanbe okr.: 18.X.1946, Ivanov A. coll., det. Blagoveshtchensky.
15♀, 3♂, 4N, ex Galerida cristata (L., 1758) (Passeriformes: Alaudidae), Crested Lark. Tajikistan. Zap. Tigrovaya Balka: 1N (slide no. 143), 29.IV.1934, and 13♀, 2♂, 4N (slides no. 140–142, 144–148), 9.II.1941, Sosnina E. coll., det. Blagoveshtchensky; Kirovobad: 1♀, 1♂ (slide no. 150), 29.VI.1932, Shtrom Zh. coll., det. Blagoveshtchensky; Saray-Kamar: 1♀ (slide no. 149), 29.VI.1932, Pospelova M. coll., det. Blagoveshtchensky.
Remarks: Chewing lice on C. a. acutirostris and G. cristata in Tajikistan have been recorded [3]. The record of R. serratus ex C. a. acutirostris was not provided in the checklist by Price et al. [19]. Ricinus serratus is known from 14 species of birds from three families of which nine species belong to the family Alaudidae [19]. The world checklist [19] included two hosts from the genus Calandrella and besides C. a. acutirostris, the records for Calandrella cheleensis (Swinhoe, 1871) recorded by Mey [15] were missed. Mey had found only two nymphs [15] and that could possibly be the reason for excluding the record from the checklist. Nevertheless, even juvenile instars of R. serratus are easily distinguishable from other Ricinus species by the presence of unique serrated pleural nodi. We suggest C. a. acutirostris and C. cheleensis be considered as valid hosts of R. serratus.
Ricinus subdiffusus Nelson, 1972
Ricinus subdiffusus Nelson, 1972: 97.
Type host: Spizella passerina (Bechstein, 1798) – Chipping Sparrow (Emberizidae).
Type locality: Hopland Field Station, Mendocino Co., California, USA.
Material examined: 1♀ (slide no. MV20) ex Emberiza sp. (Passeriformes: Emberizidae). Russia. Chukot. p-ov, Lavrentiya: 28.VII.1948, Ljobin coll.
Remarks: Although this species infests members of the family Emberizidae, we have found no previous records of it infesting birds of the genus Emberiza. Having just one specimen and doubtful collection techniques in the past, we suggest that R. subdiffusus not be considered as a parasite of Emberiza at this time.
Ricinus thoracicus (Packard, 1870)
Nirmus thoracicus Packard, 1870: 94.
Type host: Plectrophenax nivalis (L., 1758) – Snow Bunting (Emberizidae).
Type locality: Not recorded, USA.
Material examined: 1♀ (slide no. 157) ex P. nivalis (Passeriformes: Emberizidae), Snow Bunting. Russia. o-v Vrangelya b. Rodzhersa: 7.V.1939, Portenko coll., det. Blagoveshtchensky, slide no. 157.
Remarks: This specimen was examined and mentioned by Blagoveshtchensky [4].
Ricinus tugarinovi Blagoveshtchensky, 1951 (Fig. 1B)
Ricinus tugarinovi Blagoveshtchensky, 1951: 286.
Type host: Terpsiphone paradisi (L., 1758) – Asian Paradise-Flycatcher (Monarchidae).
Type locality: Kondara, Tajikistan.
Material examined: 1♀ (slide no. MV153) ex T. paradisi (Passeriformes: Monarchidae), Asian Paradise-Flycatcher. Tajikistan. Kondara: 6.VI.1944, Blagoveshtchensky coll., det. Blagoveshtchensky.
Remarks: Blagoveshtchensky [3] based his description of R. tugarinovi on 2♀, but he did not provide a slide number for the type specimen. Subsequently, Rheinwald [20], in his revision of the Old World species of Ricinus, re-described R. tugarinovi without examining the type material. Since the specimen examined in this study shares notes about locality and date with two specimens examined by Blagoveshtchensky [3], and inasmuch as we lack information on where the other specimen is deposited, the remaining specimen mounted on slide no. 153 is designated as a lectotype.
Ricinus vaderi Valan n. sp. (Figs. 1C; 2A–2E)
urn:lsid:zoobank.org:act:F18FF85A-2A81-4CE1-8A47-4CE5E4816708
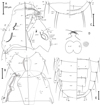 |
Figure 2. Ricinus vaderi n. sp. ♀ from Melanocorypha calandra. (A) Head, dorsoventral view: df, dorsal setae of the frons; f, ventral setae on the frons; a, setae on the temples dorsally; d, setae on the dorsum of the head; t, setae positioned dorsolaterally on temples; m, setae dorsoventrally on the marginal carinae; po, postocular setae; pa, paraantennal setae; preant., preantennal setae; ment., mental setae; pm, paramental setae; max. palp., maxillary palpi; max., maxillary setae; pal. scl., pallete sclerite; lun. n., lunar nodi; tent. n., tentorial nodi; ant. n., antennal nodi. (B) Thorax, ventrodorsal view: L, lateral prothoracic setae; pr, dorsal prothoracic setae; prst. pl., prosternal plate; prst. s., prosternal plate setae; q, setae ventrally and submarginally on the pterothorax; st. s., sternal setae; c, four pairs of setae dorsally on the pterothorax; w, series of lateral setae on the pterothorax; b, dorsal setae on the posterior margin. (C) Abdomen terminus. term. s., terminal setae of tergite IX; vps, ventral pleural setae; I, long pilose seta; i, short pilose seta; S, large spine; s, small spine (spines are on pleurites II–IV); ADF, dorsal anal fringe; AVF, ventral anal fringe. (D) Mandibles and ornamented ovoid sclerite of the nymph: ov. scl., ovoid sclerite. (E) Abdomen, dorsoventral: sen., sensilla; spir., spiracle; dps, dorsal pleural setae; ps.s., postspiracular setae; VI–VIII, tergites; sc, sternal central setae; sl, sternal lateral setae. Scale bar is 0.2 mm for all figures. |
Type host: Melanocorypha calandra (L., 1766) – Calandra Lark (Alaudidae).
Type material: Holotype ♀ (slide no. MV21) and paratype ♀ with 1N (slide no. MV22) ex M. calandra (Passeriformes: Alaudidae), Calandra Lark. Azerbaijan. Kaspiy r. Viljash-Chay: 17–18.IV.1937, Ivanov coll. Collection of chewing lice of the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia (ZISP) (Table 1).
A list of chewing lice of the genus Ricinus deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
Etymology: This species name is derived from Darth Vader, a fictional character in the Star Wars trilogy. The first author’s fiancée noticed a similarity between the head of the R. vaderi and Darth Vader’s helmet.
Authorship: Note that the authors of the new taxon are different from the authors of this paper; Article 50.1 and Recommendation 50A of the International Code of Zoological Nomenclature [12].
Description: Female (n = 2). Head wider than longer, with characteristic shape as (Fig. 2A); frons convex with rounded lateral margins, bearing dorsally pair of df setae and marginally 10 f setae; margin not continuous with that of marginal carinae. Lateral margin broadly concave. Temples expanded, outer margin curves inwardly, ending without hook-like structure; t3 one half the size of t1 and t2. Setae a1 short, each associated with two sensillae; a2 positioned marginal; a3 absent. Lunar nodi present, twice the size of tentorial nodi. Setae along antennal lappets reduced and create diastoma, numbers vary in range 6–9 with 3–4 setae associated with po setae. Preantennal setae spinose. Setae m4 same size as pa setae. Mandibles as in Figure 2D, monomorphic without finger-like extension. Ovoid sclerite round, compact with deeply pitted ornamentation. Labrum with setal pattern as in Figure 2A, pair 4 positioned slightly anteriorly to other setae in posterior row. Labium with 13 setae and pattern as in Figure 2A. Mental setae longer than maxillary setae; maxillary palpi genticuloid, extending past edge of head; maxillary plate sausage-shaped, relatively narrow. Gular plate as in Figure 2A with extensions reflexed outwardly; bearing two setae on each side.
Thorax (Fig. 2B). Setae L3 and L4 slightly pilose, one L6 seta and small seta L9. Prosternal plate without nodi, pear-shaped; prosternal setae narrowly separated (0.04 mm). Two tactile setae on coxa I. q2–q4 large and spinose, q4 sometimes absent; w series composed of four setae with anterior two spinose and twice as large as posterior two pilose setae; c1 spinose and larger than c2; c2–c4 pilose. Sternal plate as in Figure 2B bearing one moderately long posterior seta and two or three short anterior setae.
Terminal segments of abdomen as in Figures 2C and 2E. Chaetotaxy in segments II through VII follow Ricinus pattern, two setae with inner less than half the length of the outer. One pair of long setae on sternite VIII and one vulval seta. Setae in anal fringe: ADF, 41–42; AVF, 42–43. Ventral pleural chaetotaxy as in Figures 2C and 2E: II, SSS; III, sss; IV, SIS; V, iIi; VI, iIi; VII, iIi and VIII, iIi. Terminal setae as in Figure 2C with small outer pair of setae, followed by two long setae, and two pairs of small inner setae. Pleural nodi unique (Fig. 2E).
Dimensions as follows: total length = 3.73–3.82; total width = 1.11; head length = 0.72–0.73; head width = 0.83; HI = 86–87 (head index = ratio of head length to head width × 100); labrum width = 0.36–0.37; prothorax length = 0.39–0.40; prothorax width = 0.73–0.75; distance between prosternal setae = 0.039–0.040.
Diagnosis: Our specimens of R. vaderi have ornamented ovoid sclerites, present only in the diffusus, serratus and subangulatus species groups. Ricinus vaderi cannot be placed into any of these groups. In the subangulatus species group, the frontal margin is continuous with that of marginal carinae, but not in R. vaderi n. sp. or the diffusus and serratus species groups. Ricinus vaderi n. sp. has a characteristic head shape with broadly concave lateral head margins; the head is wider than the length (HI = 86–87); setae along the antennal lappets are reduced with diastoma; the setal pattern on the terminal tergite iiIIi × iIIii. In the diffusus species group, the lateral margin is almost straight; HI is 100–110, with the exception of R. thoracicus (HI = 93); there is no reduction or diastoma along the antennal lappets and the pattern on terminal tergite iIIim × miIIi (where “m” means moderately long seta). Ricinus serratus, the only member of its group, has serrated pleural nodi, the head has a postfrontal constriction, the gular plate has no posterior extensions, the a1 setae are long and there is one tactile seta on coxa I.
Remarks: Ricinus vaderi n. sp. is the second Ricinus species found on larks (Alaudidae).
Ricinus sp. 1
Material examined: 2♀ (slide no. MV23) ex Alauda arvensis (L., 1758) (Passeriformes: Alaudidae), Sky Lark. Russia. 1904, Haritonov coll.
Remarks: The condition of these specimens is poor and we were not able to identify them. These two specimens differ from R. serratus, the only known Ricinus from this host species, in not having a laterally positioned, serrated structure on the pleurites.
Ricinus sp. 2
Material examined: 2N (slide no. MV24) ex Leucosticte brandti (Passeriformes: Fringillidae), Black-headed Mountain-Finch. Kyrgyzstan. Naryn: 3.III.1913.
Remarks: These specimens are not identified to species level because of their poor condition, but they are not R. ivanovi, a known Ricinus from this host species. In contrast to R. ivanovi, the heads of these nymphs are wider than the length and have broadly concave lateral margins.
Ricinus spp. Additional samples
Material examined: 1♀ (slide no. MV25) ex Buteo lagopus (Pontoppidan, 1763) (Falconiformes, Accipitridae), Rough-legged Hawk. Russia. Okhotsk: 25.V.1936
1♀ (slide no. MV26) ex Strix aluco harmsi (Zarudny, 1911) (Strigiformes, Strigidae), Tawny Owl (Turkestan). Russia. 17.V.1947, Ivanov coll.
Remarks: Ricinidae are parasites of songbirds (Passeriformes) and hummingbirds (Apodiformes: Trochilidae); hence, Ricinus spp. 3 and 4, whose specimens differ considerably from each other probably represent two different species. They are considered as contaminants (stragglers).
Conclusions
The collection of chewing lice at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia was mainly assembled by Blagoveshtchensky in the 1930s through the 1970s. He published several surveys on the biodiversity of chewing lice in the former USSR [2–4]. We examined some specimens of Ricinus among his records. Exceptions are specimens of R. fringillae ex E. cia reported in Tajikistan [3], which have not been found in this collection. From Azerbaijan, we have recorded Ricinus vaderi n. sp. ex Melanocorypha calandra, and include previous records [2], bringing the total number of known Ricinus species in this country to three. We found only a single paper about chewing lice from Kyrgyzstan published by Kravtsova [13], who did not record any Ricinus. Our records represent the first findings of this genus in Kyrgyzstan. In addition to records of Ricinus thoracicus Packard, 1870 on Wrangel Island [4] and of Ricinus elongatus from two hosts and R. fringillae [14] in Central Ciscaucasia (northern part of the Caucasus region between the Black Sea and the Caspian Sea and within European Russia), herein we provide 10 new host-louse records in Russia. The presence of R. elongatus ex Turdus ruficollis, R. ivanovi ex Leucosticte tephrocotis, R. serratus on Calandrella acutirostris and C. cheleensis was not cited in Price et al.’s world checklist [19]. We also designated lectotype specimens for R. tugarinovi and R. ivanovi.
We consider the genus Ricinus in a restricted sense, parasitizing only Passeriformes despite the recent study by Rheinwald [21] who synonymized Trochiliphagus with Ricinus. In his revision of the genus Trochiliphagus, he declared that all 12 known species of the genus Trochiliphagus should be one single species Ricinus jimenezi.
Rheinwald based his study on investigation of only two Trochiliphagus specimens from the same host, and did not examine any of the type material. This leaves some open questions: 1. Is there indeed only one species of Trochiliphagus/Ricinus parasitizing hummingbirds or more, regardless of the correct genus? 2. Is the status of the genus Trochiliphagus valid or is it indeed part of the genus Ricinus? 3. Perhaps the genus Ricinus occurring on Passeriformes could also be divided into several genera since some species groups of this genus (dolichocephalus species group) are more similar to those of Trochiliphagus from hummingbirds than to other species groups from passerine birds (for example, arcuatus group). As Rheinwald noted, application of modern enzymatic and DNA techniques may provide the answers. Therefore, until proper DNA studies are performed or at least morphological investigations of more specimens from different hummingbird species and of course of type material for the genus Trochiliphagus, we consider Rheinwald’s hypothesis is insufficiently supported and suggest resurrection of the name Trochiliphagus.
Acknowledgments
We thank Alexandr Stekolnikov for support and assistance during a research stay of the first author at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg. We also thank Oleg Tolstenkov, who provided the literature in Russian. The authors acknowledge the recommendations of the three anonymous reviewers and the editor for their valuable comments which helped to improve the manuscript.
References
- Balát F. 1952. K poznaniu druhov rodu Ricinus De Geer 1778 (Mallophaga). Biologicky Sbornik Slovenskej Akademie vied a Umeni, 7, 155–170 (In Czech). [Google Scholar]
- Blagoveshtchensky DI. 1940. Mallophaga s ptits Tal’isha. Parazitologicheskij Zbornik, 8, 25–90 (In Russian). [Google Scholar]
- Blagoveshtchensky DI. 1951. Mallophaga of Tajikistan. Parazitologicheskij Zbornik, 13, 272–327 (In Russian). [Google Scholar]
- Blagoveshtchensky DI. 1958. Mallophaga and Anoplura from animals of the Wrangel Island. Entomologicheskoe Obozrenie, 37, 374–379 (in Russian). [Google Scholar]
- Clements FJ, Schulenberg TS, Iliff MJ, Roberson D, Fredericks TA, Sullivan BL, Wood CL. 2014. The eBird/Clements checklist of birds of the world: Version 6.9. Available at http://www.birds.cornell.edu/clementschecklist/download/ [Google Scholar]
- Emerson KC. 1972. Checklist of the Mallophaga of North America (North of Mexico). Part II. Suborder Amblycera. Deseret Test Center: Dugway, Utah. [Google Scholar]
- Enout AMJ, Lobato DNC, Diniz FC, Antonini Y. 2012. Chewing lice (Insecta, Phthiraptera) and feather mites (Acari, Astigmata) associated with birds of the Cerrado in Central Brazil. Parasitology Research, 111, 1731–1742. [CrossRef] [PubMed] [Google Scholar]
- Grube AE. 1856. Beschreibung der auf A. Th.v. Middendorff’s Sibirischer Reise gesammelten Parasiten. Buchdruckerei der Kaiserlichen Akademie der Wissenschaften: St. Petersburg. [Google Scholar]
- Halajian A, Sychra O, Luus-Powell W, Engelbrecht D, Papousek I. 2014. An annotated checklist of amblyceran chewing lice (Phthiraptera: Amblycera) from wild passerine birds (Passeriformes) in South Africa. African Entomology, 22, 762–778. [CrossRef] [Google Scholar]
- Hopkins GHE. 1942. The Mallophaga as an aid to the classification of birds. Ibis, 84, 94–106. [CrossRef] [Google Scholar]
- Ilieva MN. 2005. New data on chewing lice (Insecta: Phthiraptera) from wild birds in Bulgaria. Acta Zoologica Bulgarica, 57, 37–48. [Google Scholar]
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature, Fourth edition, International Trust for Zoological Nomenclature: London, i–xxix + 1–306. [Google Scholar]
- Kravtsova NT. 1998. Paraziticheskie chlenistonogie osnovnykh sinantropnykh ptits g. Bishkek i ego okrestnostey. (Parasitic arthropods of main synanthropic species of birds of Bishkek city and its suburbs). Summary of Ph.D. thesis, Kyrgyz State Agricultural University, Bishkek, Kyrgyzstan, 18 pp. (in Russian and English). [Google Scholar]
- Lyakhova OM, Kotti BC. 2011. Chewing lice (Mallophaga: Insecta) of birds in the Central Ciscaucasia. Entomological Review, 91, 367–376. [CrossRef] [Google Scholar]
- Mey E. 1982. Mongolische Mallophagen I. Ergebnisse der mongolischen Gemeinschaftsreise von Ornithologen aus der DDR 1979. IX, zugleich Ergebnisse der Mongolisch-Deutschen Biologischen Expedition seit 1962, Nr. 107. Mitteilungen aus dem Zoologischen Museum in Berlin, 58, 155–195. [Google Scholar]
- Mey E. 2007. Ricinus gutheili nov. spec. und weitere neue australische und neuguineische Ricinus-Arten (Insecta, Phthiraptera, Amblycera) aus dem Naturhistorischen Museum in Rudolstadt (Thüringen). Rudolstädter Naturhistorische Schriften, 14, 27–42. [Google Scholar]
- Nelson BC. 1972. A revision of the New World species of Ricinus (Mallophaga) occurring on Passeriformes (Aves). University of California Press: Berkeley. [Google Scholar]
- Oniki Y, Mey E, Willis EO. 2004. Ricinus ruficapillus n. sp. (Insecta, Phthiraptera, Amblycera, Ricinidae) – a second Ricinus species on the Rufous-capped Spinetail Synallaxis ruficapilla (Aves, Passeriformes, Furnariidae). Rudolstädter Naturhistorische Schriften, 12, 129–132. [Google Scholar]
- Price RD, Hellenthal RA, Palma RL. 2003. World checklist of chewing lice with host associations and keys to families and genera, in The Chewing Lice: World Checklist and Biological Overview. Price RD, Hellenthal RA, Palma RL, Johnson KP, Clayton DH, Editors. Illinois Natural History Survey: Champaign. p. 1–448. [Google Scholar]
- Rheinwald G. 1968. Die Mallophagengattung Ricinus De Geer, 1778. Revision der ausseramerikanischen Arten. Mitteilungen aus dem Hamburg Zoologischen Museum und Institut, 65, 181–326. [Google Scholar]
- Rheinwald G. 2007. The position of Trochiliphagus Carriker within the Ricinidae (Insecta: Phthiraptera). Bonner Zoologische Beiträge, 55, 37–46. [Google Scholar]
- Sychra O, Halajian A, Luus-Powell W, Engelbrecht D, Symes C, Papousek I. 2014. Amblyceran chewing lice (Phthiraptera: Amblycera) from wild passerines (Passeriformes) in South Africa, with a note to their phylogenetic relationships and with the description of a new species in the genus Myrsidea. African Entomology, 22, 589–601. [CrossRef] [Google Scholar]
- Sychra O, Literak I, Capek M, Havlicek M. 2007. Chewing lice (Phthiraptera) from buntings, cardinals and tanagers (Passeriformes: Emberizidae, Cardinalidae, Thraupidae) from Costa Rica, with descriptions of two new species of the genus Myrsidea (Phthiraptera: Menoponidae). Zootaxa, 1631, 57–68. [Google Scholar]
- Sychra O, Najer T, Kounek F, Capek M, Literak I. 2010. Chewing lice (Phthiraptera) on manakins (Passeriformes: Pipridae) from Costa Rica, with description of a new species of the genus Tyranniphilopterus (Phthiraptera: Philopteridae). Parasitology Research, 106, 925–931. [CrossRef] [PubMed] [Google Scholar]
- Valim MP, Lambrecht FM, Vianna ÉES. 2009. New records of chewing lice (Insecta, Phthiraptera) from birds of southern Brazil, with description of a new species. Iheringia Série Zoologia, 99, 249–258. [Google Scholar]
Cite this article as: Valan M, Sychra O & Literak I: Chewing lice of genus Ricinus (Phthiraptera, Ricinidae) deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia, with description of a new species. Parasite, 2016, 23, 7.
All Tables
A list of chewing lice of the genus Ricinus deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
All Figures
 |
Figure 1. (A) Lectotype female of Ricinus ivanovi Blagoveshtchensky, 1951. (B) Lectotype female of R. tugarinovi Blagoveshtchensky, 1951. (C) Holotype female of R. vaderi n. sp. Scale bar: 1 mm. |
| In the text | |
 |
Figure 2. Ricinus vaderi n. sp. ♀ from Melanocorypha calandra. (A) Head, dorsoventral view: df, dorsal setae of the frons; f, ventral setae on the frons; a, setae on the temples dorsally; d, setae on the dorsum of the head; t, setae positioned dorsolaterally on temples; m, setae dorsoventrally on the marginal carinae; po, postocular setae; pa, paraantennal setae; preant., preantennal setae; ment., mental setae; pm, paramental setae; max. palp., maxillary palpi; max., maxillary setae; pal. scl., pallete sclerite; lun. n., lunar nodi; tent. n., tentorial nodi; ant. n., antennal nodi. (B) Thorax, ventrodorsal view: L, lateral prothoracic setae; pr, dorsal prothoracic setae; prst. pl., prosternal plate; prst. s., prosternal plate setae; q, setae ventrally and submarginally on the pterothorax; st. s., sternal setae; c, four pairs of setae dorsally on the pterothorax; w, series of lateral setae on the pterothorax; b, dorsal setae on the posterior margin. (C) Abdomen terminus. term. s., terminal setae of tergite IX; vps, ventral pleural setae; I, long pilose seta; i, short pilose seta; S, large spine; s, small spine (spines are on pleurites II–IV); ADF, dorsal anal fringe; AVF, ventral anal fringe. (D) Mandibles and ornamented ovoid sclerite of the nymph: ov. scl., ovoid sclerite. (E) Abdomen, dorsoventral: sen., sensilla; spir., spiracle; dps, dorsal pleural setae; ps.s., postspiracular setae; VI–VIII, tergites; sc, sternal central setae; sl, sternal lateral setae. Scale bar is 0.2 mm for all figures. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


