| Issue |
Parasite
Volume 21, 2014
|
|
|---|---|---|
| Article Number | 1 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/parasite/2014001 | |
| Published online | 07 February 2014 | |
Research Article
Entamoeba histolytica-secreted cysteine proteases induce IL-8 production in human mast cells via a PAR2-independent mechanism
Les cystéine protéases secrétées par Entamoeba histolytica induisent la production d’IL-8 chez les mastocytes humains par un mécanisme indépendant de PAR2
1
Department of Environmental Medical Biology, Institute of Tropical Medicine, and Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul
120-752, Korea
2
Department of Parasitology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo, Japan
3
Department of Biological Chemistry, Weizmann Institute of Science, Rehovot
76100, Israel
* Corresponding author: myeong@yuhs.ac
Received:
30
June
2013
Accepted:
23
January
2014
Entamoeba histolytica is an extracellular tissue parasite causing colitis and occasional liver abscess in humans. E. histolytica-derived secretory products (SPs) contain large amounts of cysteine proteases (CPs), one of the important amoebic virulence factors. Although tissue-residing mast cells play an important role in the mucosal inflammatory response to this pathogen, it is not known whether the SPs induce mast cell activation. In this study, when human mast cells (HMC-1 cells) were stimulated with SPs collected from pathogenic wild-type amoebae, interleukin IL-8 mRNA expression and production were significantly increased compared with cells incubated with medium alone. Inhibition of CP activity in the SPs with heat or the CP inhibitor E64 resulted in significant reduction of IL-8 production. Moreover, SPs obtained from inhibitors of cysteine protease (ICP)-overexpressing amoebae with low CP activity showed weaker stimulatory effects on IL-8 production than the wild-type control. Preincubation of HMC-1 cells with antibodies to human protease-activated receptor 2 (PAR2) did not affect the SP-induced IL-8 production. These results suggest that cysteine proteases in E. histolytica-derived secretory products stimulate mast cells to produce IL-8 via a PAR2-independent mechanism, which contributes to IL-8-mediated tissue inflammatory responses during the early phase of human amoebiasis.
Résumé
Entamoeba histolytica est un parasite extracellulaire des tissus provoquant des colites et occasionnellement des abcès du foie chez l’homme. Les produits de sécrétion dérivés d’E. histolytica (SPs) contiennent de grandes quantités de cystéine-protéases (CPs), l’un des principaux facteurs de virulence amibiens. Bien que les mastocytes tissulaires jouent un rôle important dans la réponse inflammatoire de la muqueuse à ce pathogène, on ne sait pas si les SPs induisent l’activation des mastocytes. Dans cette étude, lorsque des mastocytes humains (cellules HMC-1) ont été stimulés avec des SPs recueillis à partir d’amibes pathogènes de type sauvage, l’expression et la production de l’interleukine IL-8 ont été significativement augmentées par rapport à des cellules incubées avec du milieu seul. L’inhibition de l’activité des CPs dans les SPs avec la chaleur ou avec E64, un inhibiteur de CP, a entraîné une réduction significative de la production d’IL-8. En outre, les SPs obtenus à partir d’amibes surexprimant l’inhibiteur de protéases à cystéine (ICP) à faible activité de CP ont montré des effets stimulants plus faibles sur la production d’IL-8 que le contrôle de type sauvage. La pré-incubation des cellules HMC-1 avec des anticorps contre le récepteur 2 activé par la protéase humaine (PAR2) n’a pas affecté la production d’IL-8 induite par SPs. Ces résultats suggèrent que les cystéine-protéases des produits de sécrétion dérivés d’E. histolytica stimulent les mastocytes pour produire de l’IL-8 par l’intermédiaire d’un mécanisme indépendant de PAR2, ce qui contribue à la réponse inflammatoire tissulaire médiée par IL-8 au cours de la phase précoce de l’amibiase humaine.
Key words: Entamoeba histolytica / Cysteine protease / Mast cell / IL-8 / Protease-activated receptor 2 (PAR2)
© Y.A. Lee et al., published by EDP Sciences, 2014
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The enteric protozoan parasite Entamoeba histolytica Schaudinn, 1903 [25] is the causative agent of human amoebiasis. E. histolytica trophozoites bind colonic mucin via amoebic Gal-lectin and subsequently cause mucin degradation and colonic epithelial cell death through apoptosis or necrosis [19, 20]. E. histolytica releases various proteases into the extracellular milieu. In particular, cysteine proteases (CPs) are abundant in E. histolytica-derived secretory products (SPs). CPs are important for host mucin and extracellular matrix (ECM) degradation [11], cytopathic effects on the host cell, and activation of IL-1 β or IL-18.
E. histolytica-induced colon cell death promotes IL-8-mediated acute tissue inflammation at the site of infection [26]. IL-8 is a potent chemoattractant and activator of neutrophils and can cause non-specific tissue damage after activation [9]. Although neutrophils are the primary target cells of IL-8, IL-8 has other effects on various kinds of cells such as endothelial cells, other granulocytes, macrophages, and mast cells. The infiltration of immune cells including neutrophils, macrophages, and mast cells at the mucosal surface was observed during E. histolytica intestinal amoebiasis, suggesting that these cells might be important in host defense against this parasite [13]. Moreover, an increase in degranulation and disruption of mast cells was reported in E. histolytica–infected mice [16], suggesting that mast cells play a role in E. histolytica–induced tissue inflammation at the inflamed site. However, the precise role of amoebic CPs in mast cell activation is poorly understood.
Mast cells contribute to the innate and adaptive host defense mechanisms through the release of an arsenal of inflammatory mediators upon activation by various stimuli [3, 12]. Tissue-residing mast cells are major players in the mucosal inflammatory response to various bacterial and parasitic infections [6]. Activated mast cells release various proinflammatory mediators including histamine, IL-6, IL-8, IL-13, prostaglandin D2, leukotriene C4 (LTC4), and tumor necrosis factor-α (TNF-α) in response to various stimuli [14]. The essential role of mast cells in the host control of infection has been shown in animal models infected with various bacterial and parasitic pathogens [2, 4, 28]. However, little is known about the effect of amoebic SPs on IL-8 secretion in mast cells.
In this study, mast cells were stimulated with E. histolytica-derived SPs from E. histolytica wild-type or mutant strains to ascertain if SPs directly induce IL-8 production. The results of this work show that amoebic CPs participate in SP-induced IL-8 production in HMC-1 cells.
Materials and methods
Reagents
Unless stated otherwise, all other chemicals were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Mouse monoclonal antibody (Ab) against human protease-activated receptor 2 (PAR2) (SAM 11) and normal mouse IgG2a were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fluorescent isothiocyanate (FITC)-labeled annexin V, PE-conjugated anti-human CD63, and PE-conjugated anti-mouse IgG1κ were purchased from BD Pharmingen (San Diego, CA, USA). Dichlorodihydrofluorescein diacetate (H2DCFDA) was purchased from Molecular Probes (Eugene, OR, USA).
Cultivation of human HMC-1 cells
The HMC-1 human mast cell line was grown in Iscove’s Modified Dulbecco’s medium (IMDM) (Invitrogen) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 0.5% penicillin-streptomycin at 37 °C in a humidified 5% CO2 atmosphere. HMC-1 cell viability, as judged by trypan blue exclusion testing, was 99%.
Culture of bone marrow-derived murine mast cells (BMMCs) and ethics
BMMCs from BALB/c mice (Orient Bio, Seoul, Korea) were obtained by culturing mouse femoral bone marrow cells in RPMI containing 10% (v/v) heat-inactivated fetal bovine serum, 0.5% penicillin-streptomycin, 50 μM β-mercaptoethanol, and 20 ng/mL IL-3 (PeproTech, Rocky Hill, NJ, USA) for 4 weeks. At that time, the cells were > 98% c-kithigh FcεRIhigh, according to flow cytometric analysis using PE anti-mouse FcεRIa (MAR-1) (eBioscience, San Diego, CA, USA) and FITC anti-mouse c-kit/CD117 (2D8) (eBioscience, San Diego, CA, USA). The present study was approved by the Yonsei University Animal Research Ethics Committee (No. 2013-0297).
Construction of E. histolytica cell lines overexpressing a GFP-tagged inhibitor of cysteine protease 1 (ICP1+/+)
To create the ICP1-overexpressing E. histolytica cell line (ICP1+/+), a full-length E. histolytica ICP1 gene was amplified by PCR from cDNA using sense and antisense oligonucleotides containing appropriate restriction sites (underlined) at the 5′-end: icp1 5′-aatcccgggATGTCATTAACTGAAGATAATAACAACACAAC-3′ (SmaI); and 5′-gggctcgagTTACTGGACATTAACTTTTAAAGTAAAAG-3′ (XhoI). The PCR-amplified DNA fragments were digested and ligated into the same restriction sites of the overexpression vector, pKT-MG. This vector allows for the expression of a gene of interest as a N-terminal fusion with GFP. The plasmids were introduced into E. histolytica HM-1:IMSS trophozoites by liposome-mediated transfection as previously described [22], and stable transformants were cultured in medium containing 8 μg/mL G418 (for ICP1+/+ and pKT-MG as a vector control).
Cultivation of E. histolytica trophozoites and preparation of secretory products
Trophozoites of the E. histolytica virulent HM-1:IMSS strain, the avirulent E. histolytica Rahman strain, and the hypo-CP strain ICP1+/+ were grown at 37 °C in TYI-S-33 medium as described previously [10]. After cultivation for 48–72 h, logarithmic growth phase trophozoites were harvested by incubation on ice for 10 min, followed by centrifugation at 1000 rpm at 4 °C for 5 min. To collect SPs, trophozoites from the various strains were incubated in Hanks’ balanced salt solution (GIBCO Laboratories, Grand Island, NY, USA) for 2 h at 37 °C at a final concentration of 1 × 107 amoebae per mL. The viability of Entamoeba trophozoites after incubation in Hank’s balanced salt solution was 99% as determined by the trypan blue exclusion assay. Protein concentration was measured by the BCA protein assay using bovine serum albumin as a standard.
Measurement of cell viability and cell death
HMC-1 cell viability and cell death were measured by trypan blue and annexin V/propidium iodide (PI) double staining, respectively. HMC-1 cells (5 × 105 cells/sample) stimulated with SPs were incubated for 2 h at 37 °C in a humidified CO2 incubator (5% CO2 and 95% air). After incubation, the reaction was stopped by brief centrifugation, and the cells were washed with cold PBS twice and stained with FITC-conjugated annex in V and 1 μg/mL PI. Flow cytometric analysis was performed with a FACScan on at least 10,000 cells from the host cell fraction.
Measurement of exocytosis in HMC-1 cells or BMMCs
To elucidate the exocytosis of HMC-1 cells or BMMCs induced by the SPs, HMC-1 cells or BMMCs (5 × 105 cells/sample) were incubated with SP (30 or 100 μg/mL) for 2 h, or platelet-activating factor (PAF) (5 μM) for 1 h or phorbol 12-myristate 13-acetate (PMA) for 2 h. For positive control, BMMCs were treated with monoclonal anti-DNP IgE (250 ng/mL) for 4 h, and then the cells were challenged with DNP-HSA (250 ng/mL) for 1 h to trigger mast cell degranulation. After incubation, the cells were washed twice with washing buffer (0.1% sodium azide and 1% FBS in PBS) and stained with PE-conjugated anti-human or anti-mouse CD63. PE-conjugated anti-mouse IgG1 or anti-rat IgG2a was used as an isotype control. A flow cytometric analysis was performed using a FACScan on at least 10,000 cells from each sample.
Quantitative real-time PCR for IL-8 mRNA expression
HMC-1 cells (1 × 106/sample) were incubated with or without SPs for 30 min at 37 °C in a CO2 incubator. After incubation, total RNA was obtained from HMC-1 cells (1 × 106/sample) incubated with or without SPs using TRIzol reagent (Invitrogen Corporation, Carlsbad, CA, USA) and was reverse-transcribed using a ProSTAR first-strand RT-PCR kit (Stratagene, La Jolla, CA, USA). Obtained cDNA was amplified using a SYBR® Green PCR Master Mix. The primers used were as follows: human IL-8; 5′-TCT GCA GCT CTG TGT GAA GGT G-3′ and 5′-AAT TTC TGT GTT GGC GCA GTG-3′, human GAPDH; 5′-GAA GGT GAA GGT CGG AGT C-3′ and 5′-GAA GAT GGT GAT GGG ATT TC-3′. IL-8 gene expression was analyzed using the Applied Biosystems 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The relative amount of mRNA for the genes of interest was determined by subtracting the threshold cycle (Ct) values for the gene from the Ct value for the internal control gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ΔCt). Each measurement of a sample was performed in triplicate. The data represent IL-8 mRNA fold induction.
IL-8 ELISA
For measurement of IL-8 production, HMC-1 cells (5 × 105/well) were seeded in 24-well tissue culture plates and then directly incubated for the indicated times with or without native or modified SPs for 12 h at 37 °C in a CO2 incubator. After incubation, culture supernatants were collected from HMC-1 cells. To evaluate the involvement of PAR2 in SP-induced IL-8 production, HMC-1 cells (5 × 105/well) were preincubated with anti-PAR2 (10 μg/mL) or mouse IgG2a (10 μg/mL) for 30 min. After preincubation, the cells were incubated for the indicated times with or without native SPs for 12 h at 37 °C in a CO2 incubator. Then, culture supernatants were collected from HMC-1 cells, and the amount of IL-8 production was measured with a specific human IL-8 screening kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Measurement of cysteine protease activity
Native SPs obtained from wild-type amoebae were pretreated with or without protease inhibitor (E64 or PMSF) or heat-modified SPs (100 °C for 10 min), and then CP assays were performed. In addition, ICP1+/+, vector control, or Rahman-derived native SPs were also measured for CP activity. Briefly, E. histolytica trophozoites (4 × 105/well) were incubated in 100 mL Opti-MEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 137 mM cysteine and 19 mM ascorbic acid, pH 6.8 in a 96-well tissue culture plate at 37 °C for 1 h. After incubation, the culture supernatant for secreted CP activity was collected and remaining trophozoites for intracellular CP activity were obtained by centrifugation. CP activity was measured according to the cleavage of a z-Arg-Arg-pNA∙2 HCl synthetic peptide substrate, which was monitored spectrophotometrically as described previously [21, 23].
Measurement of ROS generation in HMC-1 cells
Intracellular ROS accumulation in HMC-1 cells was measured by staining cells with the green fluorescence probe H2DCFDA, which is rapidly oxidized to highly fluorescent DCF in the presence of intracellular H2O2, and analyzed spectrofluorometrically (model Axiovert 200). Briefly, HMC-1 cells (1 × 105 cells/sample) were preloaded with 5 μM H2DCFDA for 30 min and washed twice with culture medium. The washed cells were incubated with or without SPs or PAF for up to 30 min at 37 °C in a CO2 incubator. The production of intracellular ROS was determined on a Perkin Elmer LS50B spectrofluorometer using excitation and emission wavelengths of 485 and 530 nm, respectively. All background fluorescence was subtracted using the appropriate controls.
Statistical analysis
All reactions were performed in triplicate measurements of each experiment. Results are presented as the mean ± SEM of 3 to 6 independent experiments. One-way analysis of variance (ANOVA) was performed using the statistical software package SPSS version 20 for Windows. The post hoc comparisons of means from different groups were made by the Bonferroni post hoc test. Values were considered statistically significant when the p-value ≤ 0.05.
Results
E. histolytica-derived secretory products do not induce mast cell degranulation
HMC-1 cells were treated with various concentrations (30 or 100 μg/mL) of SPs for 2 h to examine whether SPs could induce mast cell activation. CD63 expression is generally used as a surface marker for degranulation via exocytosis in mast cells [24]. When HMC-1 cells or BMMCs were treated with 100 μg/mL SP for 2 h, the percentage of CD63-positive cells was 8.1 ± 1.7 and 4.0 ± 0.6, respectively. However, HMC-1 cells treated with 5 μM PAF or BMMCs treated with DNP-HSA as a positive control showed a marked increase in CD63 expression (Figure 1A and 1B). At that time, there was no difference in cell viability after incubation with SPs for 2 h compared with the results for HMC-1 cells (Figure 1C) or BMMCs (data not shown) incubated with medium alone.
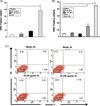 |
Figure 1. E. histolytica-derived secretory products (SPs) of pathogenic amoebae do not induce cell death or exocytosis in mast cells. (A) Percentage of CD63-positive cells in HMC-1 cells incubated with SPs or platelet-activating factor (PAF) (5 μM). (n = 3) (B) Percentage of CD63-positive cells in bone marrow-derived murine mast cells incubated with SPs, phorbol 12-myristate 13-acetate (PMA) (50nM) or DNP-HSA (250 ng/mL). (n = 4). (C) Flow cytometry analysis of HMC-1 cell death after stimulation with or without SPs. (n = 4). All reactions were performed in triplicate measurements of each experiment. All data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (**p < 0.01). |
Stimulation with secretory products increased IL-8 mRNA expression and protein secretion in HMC-1 cells
To examine whether SPs could induce secretion of IL-8 in HMC-1 cells, real-time PCR and ELISA analyses were performed. The real-time PCR analysis revealed that HMC-1 cells stimulated with SPs for 30 min resulted in a 3- to 4-fold increase in IL-8 mRNA as compared with cells treated with medium alone (Figure 2A). As shown in Figure 2B, SP-stimulated HMC-1 cells released IL-8 protein in a dose-dependent manner, and HMC-1 cells stimulated with SPs for 12 h resulted in a 1.7- and 2.4-fold increase in IL-8 protein at 30 and 100 μg/mL SP, respectively, compared with medium-treated cells. There was no difference in the number of annexin V-positive HMC-1 cells after incubation with SPs for 12 h compared with the results for cells incubated with medium alone (data not shown). Interestingly, SPs from the non-pathogenic Rahman strain showed a 30% reduction compared with SPs from HM-1:IMSS amoebae (Figure 2C).
 |
Figure 2. E. histolytica-derived secretory products (SPs) from the pathogenic wild-type HM-1:IMSS strain induce IL-8 gene expression and protein secretion in HMC-1 cells. (A) Increased IL-8 mRNA expression in HMC-1 cells induced by SPs. (n = 3) (B) IL-8 production in SPs-stimulated HMC-1 cells. (n = 6) (C) Comparison of IL-8 production in HMC-1 cells stimulated with SPs obtained from wild-type or non-pathogenic Rahman strains. (n = 4). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05, **p < 0.01). |
Cysteine protease activity is required for IL-8 production in HMC-1 cells induced by secretory products
To determine if amoebic CPs obtained from wild-type HM-1:IMSS SPs are responsible for SP-triggered IL-8 production in mast cells, modified SPs were incubated with HMC-1 cells. As shown in Figure 3A, the IL-8 production induced by SPs was abolished by pretreatment of the SPs with heat, suggesting that Entamoeba-secreted heat-labile protein components may participate in IL-8 production in HMC-1 cells. SP-induced IL-8 production in HMC-1 cells was significantly reduced by pretreatment of the SPs with the cysteine protease inhibitor E64c. Next, we compared the CP activity of native or modified SPs by treatment with specific protease inhibitors. As shown in Figure 3B, cysteine protease inhibitor E64c-treated SPs and heat-treated SPs from the wild-type significantly reduced CP activity as compared with untreated SPs. In contrast, serine protease inhibitor PMSF-treated SPs had no inhibitory effect on CP activity. To demonstrate that amoebic CP is responsible for SP-triggered IL-8 production in mast cells, we observed the IL-8 production in HMC-1 cells by SPs from the ICP1+/+ strain. SPs derived from the ICP1+/+ strain resulted in a decrease in IL-8 production compared with its transfectant control (Figure 4A). In addition, a marked reduction of CP activity was observed in SPs obtained from the ICP1+/+ strain compared with its vector control amoebae (Figure 4B).
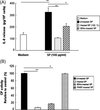 |
Figure 3. Amoebic cysteine protease activity is required for IL-8 production in HMC-1 cells induced by E. histolytica-derived secretory products (SPs). (A) IL-8 production in HMC-1 cells stimulated with SPs from native or modified wild-type SPs either heat-treated (100 °C, 10 min) or treated with 25 μM protease inhibitor (E64c). (n = 4) (B) CP activity from native or modified wild-type SPs with heat- (100 °C, 10 min) or 25 μM protease inhibitor (E64c or PMSF) treatment were measured using z-Arg-Arg-pNA·2 HCl as a substrate. The level of activity is shown as a percentage relative to the control. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05. **p < 0.01). |
 |
Figure 4. E. histolytica-derived secretory products (SPs) from the inhibitor of cysteine protease 1 (ICP1+/+) strain show a reduction in IL-8 protein secretion and cysteine protease activity in HMC-1 cells. (A) IL-8 production in HMC-1 cells stimulated with SPs from the ICP1+/+ or vector control strain. (n = 4) (B) The CP activity of SPs from the ICP1+/+ or vector control strain was measured using z-Arg-Arg-pNA·2 HCl as a substrate. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05). |
Secretory products-induced IL-8 production in HMC-1 cells occurs through a PAR2- and ROS-independent mechanism
Next, we investigated whether PAR2, which is a known G-coupled receptor, is involved in IL-8 production induced by SPs. HMC-1 cells were preincubated with monoclonal Ab (10 μg/mL) to PAR2 or the isotype control IgG2a for 30 min at room temperature and subsequently incubated for 12 h with or without SPs. As shown in Figure 5A, Ab to PAR2 did not inhibit SP-induced IL-8 production in HMC-1 cells. Next, we investigated whether ROS were involved in the SP-induced IL-8 production in HMC-1 cells. As shown in Figure 5B, intracellular ROS were not detected in SP-stimulated HMC-1 cells within 30 min. In contrast, intracellular ROS levels were strongly increased in HMC-1 cells stimulated with PAF as a positive control.
 |
Figure 5. E. histolytica-derived secretory product (SP)-induced IL-8 production in HMC-1 cells occurs through a protease-activated receptor 2 (PAR2) or ROS-independent mechanism. (A) The effect of anti-PAR2 on SP-stimulated IL-8 production. (n = 4) (B) The effect of SPs on induction of ROS generation at 30 min in HMC-1 cells. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the result of comparisons with the control (*p < 0.05). |
Discussion
In this study, our results show that amoebic CP participates in IL-8 production through a PAR2-independent pathway in HMC-1 cells. E. histolytica-derived SPs containing serine or amoebic CPs have been shown to constantly secrete CPs into the extracellular environment [1, 18]. The SPs of the Rahman and ICP1+/+ strains resulted in a significant decrease in IL-8 production in HMC-1 cells compared with the SPs of wild-type Entamoeba, suggesting that a reduction in CP activity may result in a significant reduction of SP-induced IL-8 production in HMC-1 cells. Our data indicate that amoebic CP might be involved in IL-8 production in HMC-1 cells.
Recent work has shown that E. histolytica SPs can markedly increase IL-8 mRNA expression and protein production in colonic epithelial cells [9]. In addition, recent studies have provided evidence that chemokines such as IL-8 are crucial mediators in inflammation and in tissue injury in intestinal inflammation. IL-8 is a small, 8- to 11-kDa secreted protein that may participate in immune and inflammatory responses through chemoattraction and activation of neutrophils or leukocytes [1]. E. histolytica invades the intestinal mucosa and causes amoebic colitis and severe ulceration. Analysis of the inflammatory response during intestinal amoebiasis in human and animal models of the disease has revealed an important regulatory role for chemokines and cytokines. Recruitment and activation of inflammatory cells are modulated by secreted amoebic factors. SPs contain many components including cysteine protease (CP), serine protease, other proteases, phosphatases and prostaglandin E2 (PGE2).
In our preliminary experiment, we got IL-8 results of HMC-1 cells stimulated with five various concentrations (0, 10, 30, 100, and 200 μg/mL) of SP. However, no effect of 10 μg/mL of SP on IL-8 production was observed. In addition, the highest concentration of SP (200 μg/mL) showed a cytotoxic effect on HMC-1 cells (about 10% of cells were dead for 12 h), although the amount of IL-8 release in SP (200 μg/mL)-stimulated cells showed a similar level compared with the result of stimulation with 100 μg/mL (data not shown). As a result, we chose 30 and 100 μg/mL concentrations for all experiments.
In the present study, the amount of IL-8 production in HMC-1 cells induced by heat-treated SPs (100 °C for 10 min) abrogated IL-8 production, suggesting that heat-labile proteins participate in IL-8 production in HMC-1 cells. Interestingly, this result is consistent with the fact that the RGD motif in pro-mature CP5 (PCP) binds to integrin of colon cells and induces NF-kB-mediated IL-8 production in Caco-2 cells [15]. In addition, SPs from the Rahman strain resulted in a significant decrease in IL-8 production in HMC-1 cells, whereas IL-8 production by the ICP1+/+ strain, which is deficient in CP, was slightly diminished. This result is in agreement with the report that the Rahman strain decreased CP expression. According to a previous report [9], PGE2 participation in SP-induced IL-8 production was demonstrated in colon cells, where boiled amoebic secretory product (100 °C for 30 min) abolished SP-stimulated IL-8 production. However, involvement of the lipid mediator PGE2 in SP-stimulated IL-8 production in HMC-1 cells was not investigated in this study.
There is no information on how SPs can induce mast cell activation such as IL-8 release. G-coupled receptors or Toll-like receptors (TLR) residing on the mast cell surface may act as the biological sensor for various infectious agents during the process of mast cell activation. For example, certain proteases, including serine protease and trypsin, are signaling molecules that regulate cells by cleaving and triggering PARs [27]. Accordingly, involvement of the PAR2 receptor that is activated by serine protease and occasionally by cysteine protease was tested. In particular, PAR2 is closely related to inflammation [8]. Although SPs contain many kinds of proteases including serine, cysteine and aspartic proteases, SP-induced IL-8 production in mast cells did not occur via the PAR2 receptor. Also, it is well known that TLRs act as biological sensors of various infectious agents (i.e., viruses, bacteria, or fungi) or their products (such as lipopolysaccharide, lipoteichoic acid, and peptidoglycan) and are expressed by various innate immune cells (i.e., macrophages, neutrophils, or dendritic cells) [7, 17]. In addition to recognizing external dangers, TLRs also regulate the immune response by recognizing endogenously produced danger signals including necrotic cells, heat shock proteins, or ECM breakdown products [5]. As such, TLRs may participate in SP-induced mast cell activation; however, more experimentation is needed to investigate TLR involvement in response to SPs.
In conclusion, we have demonstrated that CPs present in SPs contribute to IL-8 production in human mast cells. Additionally, non-pathogenic Rahman and the CP-deficient ICP1+/+ strains showed a decrease in IL-8 production, suggesting the involvement of amoebic CP in the host cell response induced by E. histolytica infection, helping us to understand the mechanism of pathogenesis in E. histolytica.
Acknowledgments
We would like to thank Myung-hee Yi for her help with purification of BMMCs. This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (number 2009-0070766).
References
- Ajuebor MN, Swain MG. 2002. Role of chemokines and chemokine receptors in the gastrointestinal tract. Immunology, 105, 137–143. [CrossRef] [PubMed] [Google Scholar]
- Askenase PW. 1977. Immune inflammatory responses to parasites: the role of basophils, mast cells and vasoactive amines. American Journal of Tropical Medicine and Hygiene, 26, 96–103. [Google Scholar]
- Beaven MA. 2009. Our perception of the mast cell from Paul Ehrlich to now. European Journal of Immunology, 39, 11–25. [Google Scholar]
- Befus AD, Bienenstock J. 1982. Immunity to infectious agents in the gastrointestinal tract. Journal of the American Veterinary Medical Association, 181, 1066–1068. [PubMed] [Google Scholar]
- Beg AA. 2002. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends in Immunology, 23, 509–512. [CrossRef] [PubMed] [Google Scholar]
- Bischoff SC, Krämer S. 2007. Human mast cells, bacteria, and intestinal immunity. Immunological Reviews, 217, 329–337. [CrossRef] [PubMed] [Google Scholar]
- Cook DN, Pisetsky DS, Schwartz DA. 2004. Toll-like receptors in the pathogenesis of human disease. Nature Immunology, 5, 975–979. [CrossRef] [PubMed] [Google Scholar]
- Déry O, Corvera CU, Steinhoff M, Bunnett NW. 1998. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. American Journal of Physiology, 274, C1429–C1452. [Google Scholar]
- Dey I, Chadee K. 2008. Prostaglandin E2 produced by Entamoeba histolytica binds to EP4 receptors and stimulates interleukin-8 production in human colonic cells. Infection and Immunity, 76, 5158–5163. [CrossRef] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Transactions of the Royal Society of Tropical Medicine and Hygiene, 72, 431–432. [CrossRef] [PubMed] [Google Scholar]
- Espinosa-Cantellano M, Martínez-Palomo A. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clinical Microbiology Reviews, 13, 318–331. [CrossRef] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. 2005. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annual Review of Immunology, 23, 749–786. [CrossRef] [PubMed] [Google Scholar]
- Ghosh PK, Ventura GJ, Gupta S, Serrano J, Tsutsumi V, Ortiz-Ortiz L. 2000. Experimental amebiasis: immunohistochemical study of immune cell populations. Journal of Eukaryotic Microbiology, 47, 395–399. [CrossRef] [Google Scholar]
- Hart PH. 2001. Regulation of the inflammatory response in asthma by mast cell products. Immunology and Cell Biology, 79, 149–153. [CrossRef] [PubMed] [Google Scholar]
- Hou Y, Mortimer L, Chadee K. 2010. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFkappaB-mediated pro-inflammatory responses. Journal of Biological Chemistry, 285, 35497–35504. [CrossRef] [Google Scholar]
- Im KI, Hwang HK, Soh CT. 1975. Behaviour of mast cells in Mice in the course of Entamoeba histolytica infection by strains. Korean Journal of Parasitology, 13, 115–122. [CrossRef] [Google Scholar]
- Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nature Immunology, 5, 987–995. [CrossRef] [PubMed] [Google Scholar]
- Laughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA. 2004. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infection and Immunity, 72, 5349–5357. [CrossRef] [PubMed] [Google Scholar]
- Leippe M, Ebel S, Schoenberger OL, Horstmann RD, Müller-Eberhard HJ. 1991. Pore-forming peptide of pathogenic Entamoeba histolytica. Proceedings of the National Academy of Sciences of the United States of America, 88, 7659–7663. [CrossRef] [PubMed] [Google Scholar]
- McCoy JJ, Mann BJ, Petri WA Jr. 1994. Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infection and Immunity, 62, 3045–3050. [PubMed] [Google Scholar]
- Mitra BN, Saito-Nakano Y, Nakada-Tsukui K, Sato D, Nozaki T. 2007. Rab11B small GTPase regulates secretion of cysteine proteases in the enteric protozoan parasite Entamoeba histolytica. Cellular Microbiology, 9, 2112–2125. [CrossRef] [PubMed] [Google Scholar]
- Nozaki T, Asai T, Sanchez LB, Kobayashi S, Nakazawa M, Takeuchi T. 1999. Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar. Regulation and possible function of the cysteine biosynthetic pathway in Entamoeba. Journal of Biological Chemistry, 274, 32445–32452. [CrossRef] [Google Scholar]
- Saito-Nakano Y, Mitra BN, Nakada-Tsukui K, Sato D, Nozaki T. 2007. Two Rab7 isotypes, EhRab7A and EhRab7B, play distinct roles in biogenesis of lysosomes and phagosomes in the enteric protozoan parasite Entamoeba histolytica. Cellular Microbiology, 9, 1796–1808. [CrossRef] [PubMed] [Google Scholar]
- Schäfer T, Starkl P, Allard C, Wolf RM, Schweighoffer T. 2010. A granular variant of CD63 is a regulator of repeated human mast cell degranulation. Allergy, 65, 1242–1255. [CrossRef] [PubMed] [Google Scholar]
- Schaudinn F. 1903. Untersuchungen ueber die Fortpflanzung einiger Rhizopoden. Arbeiten aus dem Kaiserlichen Gesundheitsamte, 19, 547–576. [Google Scholar]
- Seydel KB, Stanley SL Jr. 1998. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor α-dependent pathway of apoptosis. Infection and Immunity, 66, 2980–2983. [PubMed] [Google Scholar]
- Trejo J. 2003. Protease-activated receptors: new concepts in regulation of G protein-coupled receptor signaling and trafficking. Journal of Pharmacology and Experimental Therapeutics, 307, 437–442. [CrossRef] [Google Scholar]
- Wedemeyer J, Tsai M, Galli SJ. 2000. Roles of mast cells and basophils in innate and acquired immunity. Current Opinion in Immunology, 12, 624–631. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Lee YA, Nam YH, Min A, Kim KA, Nozaki T, Saito-Nakano Y, Mirelman D & Shin MH: Entamoeba histolytica-secreted cysteine proteases induce IL-8 production in human mast cells via a PAR2-independent mechanism. Parasite, 2014, 21, 1.
All Figures
 |
Figure 1. E. histolytica-derived secretory products (SPs) of pathogenic amoebae do not induce cell death or exocytosis in mast cells. (A) Percentage of CD63-positive cells in HMC-1 cells incubated with SPs or platelet-activating factor (PAF) (5 μM). (n = 3) (B) Percentage of CD63-positive cells in bone marrow-derived murine mast cells incubated with SPs, phorbol 12-myristate 13-acetate (PMA) (50nM) or DNP-HSA (250 ng/mL). (n = 4). (C) Flow cytometry analysis of HMC-1 cell death after stimulation with or without SPs. (n = 4). All reactions were performed in triplicate measurements of each experiment. All data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (**p < 0.01). |
| In the text | |
 |
Figure 2. E. histolytica-derived secretory products (SPs) from the pathogenic wild-type HM-1:IMSS strain induce IL-8 gene expression and protein secretion in HMC-1 cells. (A) Increased IL-8 mRNA expression in HMC-1 cells induced by SPs. (n = 3) (B) IL-8 production in SPs-stimulated HMC-1 cells. (n = 6) (C) Comparison of IL-8 production in HMC-1 cells stimulated with SPs obtained from wild-type or non-pathogenic Rahman strains. (n = 4). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05, **p < 0.01). |
| In the text | |
 |
Figure 3. Amoebic cysteine protease activity is required for IL-8 production in HMC-1 cells induced by E. histolytica-derived secretory products (SPs). (A) IL-8 production in HMC-1 cells stimulated with SPs from native or modified wild-type SPs either heat-treated (100 °C, 10 min) or treated with 25 μM protease inhibitor (E64c). (n = 4) (B) CP activity from native or modified wild-type SPs with heat- (100 °C, 10 min) or 25 μM protease inhibitor (E64c or PMSF) treatment were measured using z-Arg-Arg-pNA·2 HCl as a substrate. The level of activity is shown as a percentage relative to the control. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05. **p < 0.01). |
| In the text | |
 |
Figure 4. E. histolytica-derived secretory products (SPs) from the inhibitor of cysteine protease 1 (ICP1+/+) strain show a reduction in IL-8 protein secretion and cysteine protease activity in HMC-1 cells. (A) IL-8 production in HMC-1 cells stimulated with SPs from the ICP1+/+ or vector control strain. (n = 4) (B) The CP activity of SPs from the ICP1+/+ or vector control strain was measured using z-Arg-Arg-pNA·2 HCl as a substrate. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the results of comparisons with the controls (*p < 0.05). |
| In the text | |
 |
Figure 5. E. histolytica-derived secretory product (SP)-induced IL-8 production in HMC-1 cells occurs through a protease-activated receptor 2 (PAR2) or ROS-independent mechanism. (A) The effect of anti-PAR2 on SP-stimulated IL-8 production. (n = 4) (B) The effect of SPs on induction of ROS generation at 30 min in HMC-1 cells. (n = 3). All reactions were performed in triplicate measurements of each experiment. Data are presented as the mean ± SEM of at least three independent experiments. The asterisks indicate the result of comparisons with the control (*p < 0.05). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


