| Issue |
Parasite
Volume 18, Number 3, August 2011
|
|
|---|---|---|
| Page(s) | 261 - 269 | |
| DOI | https://doi.org/10.1051/parasite/2011183261 | |
| Published online | 15 August 2011 | |
Original contribution
The steppe species of gastrointestinal nematodes of small ruminants, with a focus on Marshallagia: climate as a key determinant
Les espèces de nématodes gastro-intestinaux des petits ruminants de la steppe, avec une attention particulière pour Marshallagia : le climat comme déterminant essentiel
1
Département des Sciences biologiques, Université de Batna, Algérie
2
Laboratoire de Parasitologie, Département des Sciences vétérinaires, Université Mentouri, Constantine, Algérie
3
INRA, Infectiologie animale et Santé publique 213, 37380 Nouzilly, France
* Correspondence: Jacques Cabaret. Tel.: 33 (0)2 47 42 77 68 – Fax: 33 (0)2 47 42 77 74. E-mail: cabaret@tours.inra.fr
Received:
2
November
2010
Accepted:
16
May
2011
We intended to relate the geographic distribution of ruminant gastrointestinal nematodes in relation to steppe climate (and vegetation). Data are either from literature or from newly acquired/ available results. Simple or more sophisticated meteorological indices were used to characterize the climate. Regression analyses were used to correlate climatic factors and presence of endoparasites from steppe areas. The distribution of one (Marshallagia) out of five endoparasite genera was concentrated mostly in steppic areas whereas other species were found also in other areas. In wild hosts the distribution of Marshallagia was much larger from Sptizberg to New World (northern territories in Canada or extreme south of America). In domestic small ruminants the presence of Marshallagia was identified more frequently and constantly in the area of original domestication and its early diffusion (from Northern Africa to Kashmir, Caucasia). The distribution of this parasite was correlated to low rainfalls which were not the case for all other endoparasites. After host switch (reindeer or south America camelids), it has expanded in other climatic areas, either colder or dryer.
Résumé
Notre objectif est de relier la distribution géographique des nématodes gastro-intestinaux des ruminants aux caractéristiques du climat (et de la végétation) steppique. Nous avons utilisé les données de la littérature et d’autres plus récentes que nous avons acquises. Nous avons recouru à des indices simples ou sophistiqués pour caractériser le climat. Des analyses de régression ont été utilisées pour estimer les relations entre les nématodes gastro-intestinaux et les facteurs climatiques. La distribution d’un genre (Marshallagia) sur les cinq rencontrés était surtout concentrée dans les zones steppiques, alors que les autres espèces étaient également rencontrées dans d’autres régions. Chez les hôtes sauvages, la distribution était beaucoup plus large, du Spitzberg au Nouveau Monde (territoires du nord du Canada et extrême sud de l’Amérique). Chez les petits ruminants domestiques, la présence de Marshallagia a été recensée de manière plus intense et plus constante dans les zones initiales de domestication ou dans ses diffusions proches (de l’Afrique du Nord au Cachemire ou au Caucase). La distribution de ce parasite était reliée à de faibles pluies, à l’inverse de ce qui était noté pour les autres nématodes. Ce n’est que lors de captures (renne, camélidés sud américains) que ce nématode a élargi son aire de distribution, dans des zones plus froides ou plus sèches.
Key words: small ruminant / endoparasite nematode / Marshallagia / climate / steppe / host switch
Mots clés : petit ruminant / nématode endoparasite / Marshallagia / climat / steppe / capture
© PRINCEPS Editions, Paris, 2011, transferred to Société Française de Parasitologie
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
It may look surprising that parasites would display macroecological and biogeographical patterns similar to those described for free-living organisms (Gueguan, 2006). In fact one may observe in many macroparasites similar ecological patterns as observed for terrestrial or marine free-living taxa (Poulin & Morand, 2004; Perez del Olmo et al., 2009). Among the gastrointestinal parasite nematodes of herbivores, one part of the life is non-parasitic on the grass and they are clearly influenced by climatic environment (O’Connor et al., 2006). The main difference is that their life within a host, who ingested infected herbage, is regulated to some extent by host susceptibility (Gaba et al., 2006). One major difference in domesticated hosts is that they are often treated with anthelmintics, which may alter in the long run the diversity of macroparasites (Silvestre et al., 2000). A second difference in these domesticated hosts is that they are transported from one site to another by their owner, sometimes on long distances for commercial reasons or availability of food. It could then be expected that ecological traits remains unexplainable due to these confounding effects of human management.
A previous study on the importance of climate and host species on occurrence of some macroparasitic nematodes (Ostertagiinae) of ungulates (mostly domestic ones and from 585 references altogether) did show that host species and climate played apparently a similar role (18% of occurrences explained equally either by range of host species or climates) (Suarez & Cabaret, 1991). Interspecific interactions in the gastrointestinal community are limited (Cabaret & Hoste, 1998) and this may simplify the understanding of climate/host factors. One of the genera of macroparasitic nematodes, Marshallagia sp., was associated with steppic climate and with domestic sheep and goats. However we know that it may be present in muskoxen or reindeer under polar climate (Halvorsen & Bye, 1999, in the Spitzberg) and in wild ungulates in several part of mountainous Europe (Italian Alps, among others: Zaffaroni et al., 2000). The steppe climate comes under Köppen’s BS classification of climates (Viers & Vigneau, 1990). The B stands for dry climates, and the S for steppe climate. There is a huge difference between summer (up to 30 °C monthly temperature) and winter (sometimes below 0 °C monthly temperature) and the differences between day and night are also great. Some mediterranean climates (portuguese and hellenic) are not highly different from steppe climate but the number of dry-month is smaller. Steppe regions are often found in middle of continents and in the lee of mountains. Steppe vegetation may be found under different climates, and this renders the term steppe equivocal. Vegetation often consists in small xerophytic discontinuous grassland cover (opposed to prairie with continuous grass cover or savannah with tall grass). Steppe vegetation thus can be found in regions with very cold winter and hot summers (Central Asia, Eastern Europe) or under subarid mediterraneans climates (North Africa and several Middle-East countries). Thus steppe, either defined by climate or vegetation is not a very clear concept and we will substitute simple measures such as annual mean temperature or yearly rainfall in areas where Marshallagia is highly represented, since it was associated with steppe vegetation in a previous analysis (Suarez & Cabaret, 1991). Nematodirus species are also highly prevalent in steppe regions but they are also found in many different climatic areas (Morgan et al., 2006). Genus such as Haemonchus (from temperate down to tropical areas where it predominates) or Teladorsagia (not found under the tropics but well adapted to temperate or cold climates) are found under a large range of climates; Trichostrongylus genera is nearly present under any type of climate. For example, North Africa has a large part of the inland under typical steppe climate and vegetation (Slimani et al., 2010), and as expected, Marshallagia has been very frequently recorded (Morocco: Cabaret, 1984; Algeria: Bentounsi et al., 2007 and Boulkaboul & Moulaye, 2006); but other trichostrongylid nematodes such as Teladorsagia circumcincta, Haemonchus contortus, Trichostrongylus colubriformis, Trichostrongylus vitrinus, Trichostrongylus probolurus and Nematodirus filicollis are also frequently recorded (Graber, 1979).
We intend to answer to the following questions: 1) is Marshallagia associated to particular climatic characteristics such as average temperature and annual rainfalls or rather to a set geographical locations in domestic sheep and goats? 2) is Marshallagia associated with other nematode species in sheep and goats? 3) could the wild hosts infection with Marshallagia help to decipher the distribution of this species?
Materials and Methods
Parasitological Methods
Parasitological examinations concern faeces or gastrointestinal tract of necropsied hosts. The differentiation of eggs in faeces is limited to Marshallagia, Nematodirus and other gastrointestinal nematodes (MAAF), 1986). The worms collected at necropsy are identified to species (Skrjabin et al., 1954). The organs examined were abomasa (glandular stomach of ruminants) or abomasa and small intestines.
Recorded Occurrences of Marshallagia Marshalli in Literature
The occurrences were obtained from older records (Skrjabin et al., 1954) or more recent records using Web of Science – All data bases in May 2010. We also used parts of the records from Suarez & Cabaret, 1991, that were based on Helminthological Abstracts (1960-1989), Index Catalogue of Medical and Veterinary Zoology (1966-1982). It was a critical review of occurrences and the cattle occurrences were discarded since either Skrjabin et al. (1954) or ourselves in our unpublished investigations could not find Marshallagia in cattle. These occurrences are shown in Fig. 1A, B.
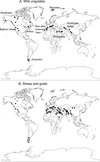 |
Fig. 1. Geographic distribution of the nematode Marshallagia marshalli in wild ungulates (A) and domestic sheep and goats (B) established on records from 1930 to 2010. |
Own Survey in Algeria
The studied farm (el Mader) is located in the North- East of Algeria (Department of Batna). The coldest month is January with 5.6 °C. The hottest month is July with 27.5 °C. Yearly rainfalls are 368 mm and the drought period extends from June to September. The climate is categorized as semi-arid with temperate winter or steppic. 30 weaned lambs were selected among the available lambs and were used as sentinels to monitor the infection. They were monthly examined for faecal egg counts and two to three lambs were necropsied monthly to identify the species of gastrointestinal nematodes (January 2008-March 2009).
Meta-Analysis of Nematofauna in Steppe Areas
The sites were selected on the basis of constant presence of Marshallagia and detailed results that encompasses several seasons. The sites were also representative of a large geographic range with Marshallagia. Nine sites or periods were investigated regarding sheep faecal egg counts (Eastearn and western Algeria, Northwestern areas of China, Center area of Kazakhstan, and four areas of Northwestern Syria) with Marshallagia prevalence ranging from 10 (Kazakhstan) to 85% (our survey in Algeria) (Table I). Necropsies of hosts were performed to identify nematodes found in abomasa in nine sites or periods (Morocco, Mongolia, Kashmir, Syria, Spain) (Table II). The percentage of Marshallagia in the abomasum nematode community ranged from 3 (year three in Morocco) to 81 (Syria 4). Necropsies of hosts on all the digestive-tract were conducted in few sites (Algeria, Kazakhstan, Turkey-Van and Syria) and prevalences are shown (Table III).
Prevalence of nematodes groups in sheep and goats based on faecal egg counts.
Proportions within the community of nematodes species of sheep and goats based on necropsies (abomasum).
Prevalences of nematode species in sheep and goats: necropsies of all gastrointestinal-tract in highly Marshallagia infected sites.
Climatological Parameters and Analysis of Data
The rainfalls (R) are below 40 cm per year in steppe climate. The rainfalls in steppe climates are related to yearly average temperatures (t in °C) in the following ways: R in cm < 2 t when dry season occurs in summer, R < (t + 14) when dry season is in winter and R < 2 (t + 7) when no season effect is observed. We used the difference between R - 2 t, R - (t + 14) or R - (2 t + 14) as an index of departure from steppe climate either in dryness (negative values) or excess of humidity (positive values) and we call it DS (departure from steppe). The lowest value was in, Syria (- 12) and the highest in León 4 (53). The De Martonne’s aridity index I is calculated as follows: I = R in mm / (t + 10) and is between 5 to 10 for dry steppe. It ranged from 7.2 (Syria) to 35.7 (León 4).
All the statistical analyses were performed using SPSS 11.5 (regressions) or MVSP 3.1. (cluster analyses based on UPGMA and Spearman rank correlations) softwares.
Results
Geographic Distribution of Marshallagia in Wild and Domestic Ungulates
The distribution of Marshallagia in wild ungulates (Fig. 1A) was relatively extended among continents but restricted to few sites. Most of the distribution was associated to mountains except in reindeer from Spitzberg. The wild ungulates disseminated Marshallagia into polar or boreal climates. The infected domestic sheep and goats (Fig. 1B) were also recorded in mountain regions either in the New World (Rocky mountains and Appalachians in North America and the Andes in South America) or in the Old World (Alps, Atlas, Taurus, Caucasus, Himalaya, and Altai). The majority of occurrences were located in Eurasia (45 out of 51 sites) of which half were in the original areas of domestication (Middle-East and Northern India).
Nematode Faunistic Similarity Between Sites and Periods
The faecal egg counts provide a first gross evaluation on the nematode fauna (Table I). The prevalence of Marshallagia ranged from 10 (Kazakhstan) to 85% (Algeria). It should be noted that it may vary much from different sites in the same country (21 to 81% in Algeria). The importance of other nematodes characterized different sites with the high proportion of H. contortus in Kashmir valley and in Mongolia, of T. vitrinus in Syria 4, of Trichostrongylus axei in Morocco and León (Table II). When considering the total fauna and prevalence of species in sites with high prevalence of Marshallagia (> 50% hosts), most of the other species were highly variable among sites (Table III). The ratios variance to mean of prevalences were low for Marshallagia and T. probolurus and were high for all the other species or for Nematodirus genus. On the UPGMA performed on the prevalences of parasites in abomasa (Fig. 2) an Eurasian group appears (Syria, Turkey, Kazakhstan, Kashmir and Mongolia). Two groups are climatically driven: one group is in relation to the subhumid climate (Morocco and León 4 in Spain) and another group corresponds to arid climate (León 1 to 3 in Spain and Batna in Algeria).
 |
Fig. 2. Prevalence of nematode species located in abomasum: data of 16 surveys in steppe and cluster analysis using UPGMA and Spearman correlation coefficient. |
Relationship Between Climate and Presence of Marshallagia and Other Nematode Species
The significant relations between prevalence and climate indicators were: Marshallagia with rainfall (rs = - 0.78; n = 16), Martonne index (rs = - 0.54) and DS (rs = - 0.60), T. circumcincta (Martonne index rs = 0.72, DS rs = 0.79; n = 16), T. axei (rainfall rs = 0.77, DS rs = 0.53; n = 16), and Nematodirus (rainfall rs = 0.73; n = 9). Rainfall was the best indicator for prevalence of Marshallagia and other nematode species. A significant linear regression was established between the prevalence of Marshallagia and rainfall (Fig. 3). Contrasting to most other nematode species, lower rainfall was associated to higher Marshallagia prevalence of infection.
 |
Fig. 3. Linear relationship between prevalence of the nematode Marshallagia marshalli and rainfall in 16 surveys in steppe. (Ln: neperian logarithm, R: regression coefficient and P probability of type 1 error). |
Discussion
Maximum co-speciation has been considered as the norm, but it must be replaced by a more general model of evolution that includes host switching to understand the geographic distribution of host parasite systems. Host-switching and geographical dispersal of parasites are common phenomena in natural host-parasite systems (Hoberg & Brooks, 2008). These events of high importance at an evolutionary scale of time may also play a role at the historical scale particularly for domestic hosts and their parasites. The geographic range of domestic herbivores has expanded enormously, as well as the number of hosts (of the original host species and also other species the parasite was in contact with). It remains however difficult to decipher the role of physical environment (climate included) and geographic location per se in the presence of domestic animals in a region. The goat may well have been domesticated a little earlier than other domestic ruminants. The ancestral wild stock exists, is the wild goat (Capra aegagrus), which is found from Anatolia to Pakistan, in the Neolithics (Moutou & Pastoret, 2010). It is probable that there were two invasions to Africa, in the 5th and in the 3rd millennium BP. Sheep were introduced to the southern Levant as fully domesticated by the end of the 9th millennium BP (Haber & Dayan, 2004) and presented similar invasions. Thus the area extending from North Africa to Pakistan and possibly Caucasus (supported by the genetic analysis of wild and domesticated sheep: Hienleder et al., 2002) constitutes the original area of domestic sheep and goats at the end of the Neolithic. The presence of the parasitic nematode Marshallagia corresponds in part to the origin of the small ruminants although it has expanded to Spain and mountain areas of several parts of Europe. It has also clearly expanded geographically through the dissemination of domestic sheep and goats. This parasite has been found in wild Ovis (prevalence 11.5% similar to domestic sheep, in the data set of Suarez & Cabaret, 1999) or Capra (prevalence 15.5% in the same data set) but was captured by reindeer, muskoxen, and several species of cervids, among which Caribou. There is thus a contradiction to the relatively limited area of Marshallagia in sheep and goats and its capabilities to invade a wide variety of hosts and climates (Fig. 1A). It is an illustration of the Beijerinck’s law “Everything is everywhere, but the environment selects” (cited in Perez del Olmo et al., 2009): “everywhere” concerns mostly wild ungulates and small ruminants and “environment selects” is particularly true for the nematode infection of worldwide distributed domestic sheep and goats.
Climate is clearly a key for the distribution of gastrointestinal nematodes of sheep and goats (O’Connor et al., 2006). Moreover, sheep and goats are present throughout the world and if distribution of parasitic nematodes was only host driven, they should be found everywhere, which is not the situation and particularly for Marshallagia. Rainfalls play a major role and temperature (at least in steppe areas) is less important. More sophisticated climatic indices (Martonne or index of departure from steppe climate) are not more efficient for prediction of nematode prevalence in a site. The relation to rainfalls was negative for Marshallagia and was positive for all other species. In fact the highest prevalence was observed in areas with low rainfall (180 to 385 mm per year). The seasonal dynamics indicates that October-November in Morocco is the period with higher intensities (Cabaret, 1984); a similar trend was noted in Algeria (our unpublished results), in Spain (Diez-Baños, 1989) as well as in Uzbekistan (Oripov, 1982). This means that infection should occur at the end of the dry-season or the very beginning of rainy season. This seasonality corroborates the climatic environment needed for Marshallagia, a relative dryness. The capability to withstand dryness is however limited: there is no development from May to September of Marshallagia in very arid steppe of Uzbekistan (Marchanov et al., 1985). The parasite community, with the exception of Marshallagia was highly variable (variance to mean ratio was high for all other specie that were largely represented) and there was no community that could be identified for steppic climate. This climate is then characterised by a single genera (Marshallagia) which is represented by very few species. In the absence of molecular genetic studies it is yet difficult to understand if there was an expansion with a relatively generalist species of parasite (able to survive in different hosts) or if there was a local genetic differentiation between parasitic isolates.
Acknowledgments
We are grateful to the University of Batna (Algeria) for funding two stays in France (INRA) to one of us (S.M) that permitted the planning of investigations and analyses of the data.
References
- Bentounsi B., Attir B., Meradi S. & Cabaret J. Repeated treatment faecal egg counts to identify gastrointestinal nematode resistance in a context of low-level infection of sheep on farms in eastern Algeria. Veterinary Parasitology,2007, 144, 104–110. [CrossRef] [PubMed] [Google Scholar]
- Boulkaboul A. & Moulaye K. Parasitisme interne du mouton de race Ouled Djellal en zone semi-aride d’Algérie. Revue d’Élevage et de Médecine Vétérinaire des Pays Tropicaux,2006, 59, 23–29. [Google Scholar]
- Cabaret J. Seasonal changes in the abomasal nematodes of naturally infected ewes in Moulay-Bouazza (Morocco). Veterinary Parasitology, 1984, 15, 47–56. [CrossRef] [PubMed] [Google Scholar]
- Cabaret J. & Hoste H. Comparative analysis of two methods used to show interspecific interactions in naturally acquired parasite nematode communities from the abomasums of ewes. Veterinary Parasitology, 1998, 76, 275–285. [CrossRef] [PubMed] [Google Scholar]
- Cai K.Z. & Bai J.L. Infection intensity of gastrointestinal nematodosis and coccidiosis of sheep raised under three types of feeding and management regimes in Ningxia Hui Autonomous Region. China. Small Ruminant Research, 2009, 85, 111–115. [CrossRef] [Google Scholar]
- Cengiz Z.T. & Değer M.S. Sheep trichostrongylidosis in Van province. Turkiye Parazitol Derg, 2009, 33, 222–226. [PubMed] [Google Scholar]
- Diez-Baños N. Estudio epidemiológico sobre los nematodos gástricos ovinos de la provincia de León, con especial referencia a Ostertagia spp. Tesis de Doctor en veterinaria, 1989, León, 364p. [Google Scholar]
- Diez-Baños N., Cabaret J. & Diez-Baños P. Interspecific interactions in naturally acquired nematode communities from sheep abomasum in relation to age of host and season in four areas of León (Spain). International Journal for Parasitology, 1992, 22, 327–334. [CrossRef] [PubMed] [Google Scholar]
- Gaba S., Gruner L. & Cabaret J. The establishment rate of a sheep nematode: revisiting classics using a meta-analysis of 87 experiments. Veterinary Parasitology, 2006, 140, 302–311. [CrossRef] [PubMed] [Google Scholar]
- Giangaspero M., Thomson E.F., Orita G., Bahhady F.A. & Gruner L. Gastrointestinal parasites and lungworms of ewes in farm flocks of North-West Syria: epidemiology and effects on productivity. Veterinaria Italiana, 1994, 30, 25–30. [Google Scholar]
- Graber M. Bibliographie des parasites internes des animaux domestiques et sauvages du Maghreb. du Sahara et de la Mauritanie. Institut d’Élevage et de Médecine Vétérinaire des Pays Tropicaux, Maisons-Alfort, France, 1979, 196 p. [Google Scholar]
- Guégan J.F. Do parasite organisms have biogeographies? Global Ecology and Biogeography, 2006, 15, 318–320. [Google Scholar]
- Haber A. & Dayan T. Analyzing the process of domestication: Hagoshrim as a case study. Journal of Archaeological Science, 2004, 31, 1587–1601. [CrossRef] [Google Scholar]
- Halvorsen O. & Bye K. Parasites, biodiversity, and population dynamics in an ecosystem in the high arctic. Veterinary Parasitology, 1999, 84, 205–227. [CrossRef] [PubMed] [Google Scholar]
- Hörchner F. Zur Helminthenfauna der Schafe in Syrien. Berlin München Tierärztliche Wochenschrift, 1964, 77, 33–36. [Google Scholar]
- Morgan E.R., Torgerson P.R., Shaikenov B.S., Usenbayev A.E., Moore A.B.M., Medley G.F. & Milner-Gulland E.J. Agricultural restructuring and gastrointestinal parasitism in domestic ruminants on the rangelands of Kazakhstan. Veterinary Parasitology, 2006, 139, 180–191. [CrossRef] [PubMed] [Google Scholar]
- Hiendleder S., Kaupe B., Wassmuth R. & Janke A. Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies. Proceedings of the Royal Society of London, Series B-Biological Sciences, 2002, 269, 893–904. [CrossRef] [Google Scholar]
- Hoberg E.P. & Brooks D.R. A macroevolutionary mosaic: episodic host-switching, geographical colonization and diversification in complex host-parasite systems. Journal of Biogeography, 2008, 35, 1533–1550. [CrossRef] [Google Scholar]
- Ministry Of Agriculture, Fisheries And Food (MAAF). Manual of Veterinary Parasitological Laboratory Techniques. Reference Book 418. HSMO Books, London, 1986, 159 p. [Google Scholar]
- Matchanov N.M., Dadaev S., Zimin Yu M., Nazarov A.N. & Nazarov A.S. Survival of the eggs and larvae of nematodes of Karakul sheep in the conditions of central Kazylkum. Kazylkum. Uzbekskii Biologicheskii Zhurnal, 1985, 5, 41–45. [Google Scholar]
- Moutou F. & Pastoret P.P. Geographical distribution of domestic animals: a historical perspective. Revue Scientifique et Technique de l’Office International des Épizooties, 2010, 29, 95–102. [Google Scholar]
- O’Connor L.J., Walkden-Brown S.W. & Kahn P. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Veterinary Parasitology, 2006, 142, 1–15. [CrossRef] [PubMed] [Google Scholar]
- Oripov A.O. Epizootiology of trichostrongylid infections in sheep in Uzbekistan. Sbornik Nauchnykh Trudov Sredneaziaticheskogo Otedeleniya VASKhNIL, 1982 9, 89–100. [Google Scholar]
- Perez-Del-olmo A., Fernandez M., Raga J.A., Kostadinova A. & Morand S. Not everything is everywhere: the distance decay of similarity in a marine host-parasite system. Journal of Biogeography, 2009, 36, 200–209. [CrossRef] [Google Scholar]
- Poulin R. & Morand S. Parasite biodiversity. Smithsonian Books, Washington, 2004, 216 p. [Google Scholar]
- Silvestre A., Chartier C., Sauvé C. & Cabaret J. Relationship between helminth species diversity, intensity of infection and breeding management in dairy goats. Veterinary Parasitology, 2000, 94, 91–105. [CrossRef] [PubMed] [Google Scholar]
- Sharkhuu T. Helminths of goats in Mongolia. Veterinary Parasitology, 2001, 101, 161–169. [CrossRef] [PubMed] [Google Scholar]
- Skrjabin K.I., Shikobalova N.P. & Shultz R.S. Trichostrongylids of animal and man. Natural Science Foundation, Department of Agriculture, Washington, 1954, 483 p. [Google Scholar]
- Slimani H., Aidoud A. & Rozé F. 30 years of protection and monitoring of a steppic rangeland undergoing desertification. Journal of Arid Environments, 2010, 74, 685–691. [CrossRef] [Google Scholar]
- Suarez V.H. & Cabaret J. Similarities between species of the Ostertagiinae (Nematoda: Trichostrongyloidea) in relation to host-specificity and climatic environment. Systematic Parasitology, 1991, 20, 179–185. [CrossRef] [Google Scholar]
- Tariq K.A., Chishti M.Z. & Ahmad F. Gastro-intestinal nematode infections in goats relative to season, host sex and age from the Kashmir valley India. Journal of Helminthology, 2010, 84, 93–97. [CrossRef] [PubMed] [Google Scholar]
- Viers G. & Vigneau J.P. Éléments de climatologie. Nathan, Paris, 1990, 224 p. [Google Scholar]
- Zaffaroni E., Manfredi M.T., Citterio C., Sala M., Piccolo G. & Lanfranchi P. Host specificity of abomasal nematodes in free ranging alpine ruminants. Veterinary Parasitology, 2000, 90, 221–230. [CrossRef] [PubMed] [Google Scholar]
All Tables
Proportions within the community of nematodes species of sheep and goats based on necropsies (abomasum).
Prevalences of nematode species in sheep and goats: necropsies of all gastrointestinal-tract in highly Marshallagia infected sites.
All Figures
 |
Fig. 1. Geographic distribution of the nematode Marshallagia marshalli in wild ungulates (A) and domestic sheep and goats (B) established on records from 1930 to 2010. |
| In the text | |
 |
Fig. 2. Prevalence of nematode species located in abomasum: data of 16 surveys in steppe and cluster analysis using UPGMA and Spearman correlation coefficient. |
| In the text | |
 |
Fig. 3. Linear relationship between prevalence of the nematode Marshallagia marshalli and rainfall in 16 surveys in steppe. (Ln: neperian logarithm, R: regression coefficient and P probability of type 1 error). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


