| Issue |
Parasite
Volume 32, 2025
|
|
|---|---|---|
| Article Number | 44 | |
| Number of page(s) | 17 | |
| DOI | https://doi.org/10.1051/parasite/2025037 | |
| Published online | 25 July 2025 | |
urn:lsid:zoobank.org:pub:894679DA-16FA-4A0B-BC11-124233FF6A11
Research Article
Two new species of Microcotylidae Taschenberg, 1879 (Platyhelminthes: Polyopisthocotyla) parasitising Diplodus capensis (Teleostei, Sparidae) off South Africa
Deux nouvelles espèces de Microcotylidae Taschenberg, 1879 (Plathelminthes : Polyopisthocotyla) parasitant Diplodus capensis (Teleostei, Sparidae) au large de l’Afrique du Sud
1
Water Research Group, Unit for Environmental Sciences and Management, North-West University, Potchefstroom 2531, South Africa
2
Department of Zoology, Swedish Museum of Natural History, Box 50007, Stockholm SE-104 05, Sweden
3
Department of Biological Sciences, Klein College of Science, University of North Carolina at Charlotte, Charlotte, North Carolina, 28223-0001, USA
* Corresponding author: nico.smit@nwu.ac.za
Received:
7
November
2024
Accepted:
10
June
2025
Microcotylids have rarely been reported along the South African coast, even though the Microcotylidae is one of the dominant polyopisthocotylan families. The present study focused on elucidating the parasite diversity of the Cape white seabream, Diplodus capensis (Smith), from various localities along the South African coast. By combining molecular and morphological techniques, two previously undescribed species of the Microcotylidae were identified. Atriaster ibamba n. sp. primarily differs from its congeners by the number and size of the hooks surrounding the genital atrium. Polylabris dassie n. sp. has a single vagina and is unique to most others of this genus by having a smaller male copulatory organ, and by the shape of this organ. This is the first report of species of Atriaster from South Africa, as well as the first report of any polyopisthocotylan from D. capensis. The present study also contributes the first genetic sequences of marine microcotylids from South Africa.
Résumé
Les Microcotylidae ont rarement été signalés le long des côtes sud-africaines, bien cette famille soit l’une des plus nombreuses des Polyopisthocotyla. La présente étude s’est attachée à élucider la diversité parasitaire du sar commun du Cap, Diplodus capensis (Smith) dans diverses localités de la côte sud-africaine. En combinant des techniques moléculaires et morphologiques, deux espèces de Microcotylidae jusque-là non décrites ont été identifiées. Atriaster ibamba n. sp. se distingue principalement de ses congénères par le nombre et la taille des crochets entourant l’atrium génital. Polylabris dassie n. sp. possède un seul vagin et se distingue de la plupart des autres espèces de ce genre par son organe copulateur mâle plus petit et par la forme de cet organe. Il s’agit de la première mention d’une espèce d’Atriaster en Afrique du Sud, ainsi que de la première mention d’un Polyopisthocotyla chez D. capensis. Cette étude fournit également les premières séquences génétiques de Microcotylidae marins d’Afrique du Sud.
Key words: Microcotylidae / Atriaster / Polylabris / Marine fish parasites / Integrative taxonomy / Life under water
Edited by: Jean-Lou Justine.
© A. Vermaak et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The diversity of polyopisthocotylans of the Microcotylidae Taschenberg 1879 hosted by marine fishes along the South African coast remains largely uncharted. To date, only a single microcotylid species, Polylabris madagascarensis Hayward, 1996 from the silver sillago, Sillago sihama (Fabricius), has been reported from this region [26]. This is noteworthy considering that the Microcotylidae is one of the largest polyopisthocotylan families, consisting of about seven subfamilies and 51 genera [4]. Given the very high biodiversity supported by the unique marine habitats along the South African coast, one would anticipate greater diversity of these parasites [62].
The Cape white seabream, Diplodus capensis (Smith) (Teleostei, Sparidae), is commonly found in a variety of near-shore habitats spanning the southern African coast from Angola to Mozambique [27]. It is popular among shore anglers and is integral in subsistence fishing, particularly in regions like southern Angola [58]. Therefore, this fish species holds both significant ecological and socioeconomic importance. Thus far, only five metazoan parasites have been described or reported from this fish host: the digeneans Holorchis pycnoporus Stossich, 1901 [9] and Proctoeces maculatus (Looss, 1901) [67]; the copepod Caligus epinepheli Yamaguti, 1936 [22]; as well as the isopods Ceratothoa famosa Hadfield, Bruce & Smit, 2014 [25], and Gnathia pilosus Hadfield, Smit & Avenant-Oldewage, 2008 [24]. Notably, no descriptions or reports of polyopisthocotylan species have been published from this fish host thus far.
During an in-depth study aimed at unravelling the diversity of metazoan parasites from D. capensis, two species of microcotylids (Prostatomicrocotylinae Yamaguti, 1968 and Atriasterinae Maillard & Noisy, 1979) were collected. A detailed morphological and molecular study revealed that the microcotylids obtained from the gills of D. capensis from South Africa represent species new to science.
Material and methods
Host and parasite collection and ethics
Sixty-eight specimens of D. capensis were collected using rod and reel from five localities along the South African coast: Koppie Alleen, De Hoop Nature Reserve (DHNR) (n = 12), Witsand (n = 3), Mossel Bay (n = 5), the Tsitsikamma section of the Garden Route National Park (TNP) (n = 29), and Chintsa East (n = 19) (Fig. 1). After being humanely killed by a combination of stunning, cranial pithing and spinal severance, the fish were subjected to full parasite screening. Sampling was conducted under the following permits: CN44-87-18289 for De Hoop Nature Reserve; RES2020/29, RES2021/49, and RES2022/44 for Witsand, Mossel Bay, and Chintsa; and MALH-K2016-005a and SMIT-NJ/2020-004 for the Tsitsikamma section of the Garden Route National Park. Ethics clearance for this research was received from the North-West University AnimCare ethics committee (NWU-00759-22-A5). Fish nomenclature and common names followed FishBase [21]. Calculations of prevalence and intensity of infection followed Bush et al. [10].
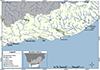 |
Figure 1 Map of sampling localities along the South African coast. Abbreviations: DHNR, De Hoop Nature Reserve; TNP, the Tsitsikamma section of the Garden Route National Park. |
Morphological methods
Polyopisthocotylans were heat-fixed without pressure in near-boiling saline and preserved immediately in 80% ethanol for parallel morphological and molecular characterisation. A total of 13 specimens were processed as hologenophores (sensu Pleijel et al. [55]). A small lateral part of the polyopisthocotylan was excised with a scalpel blade and used for molecular analyses, whereas the rest of the specimen was stained and mounted on a slide. Whole-mounts and hologenophores for morphological analyses were stained with acetic carmine, destained with diluted hydrochloric acid, dehydrated in an ethanol series (70%, 96% and 100%), cleared in clove oil and mounted in Canada balsam. Drawings were made with the aid of a Nikon Eclipse Ni-U Microscope (Nikon Instruments, Tokyo, Japan) with DIC (differential interference contrast) and a drawing tube. Drawings were scanned and digitised using Adobe Illustrator 2023. Measurements of whole-mounts and hologenophores are in micrometres (μm) and given as a range followed by the mean in parentheses. Measurements of newly characterised species and other valid species of Atriaster and Polylabris are given in the Supplementary material (Table S1).
For scanning electron microscopy, specimens were dehydrated in a graded ethanol series, followed by a graded hexamethyldisilazane (HMDS) series. Thereafter, specimens were sputter-coated with a mixture of gold and palladium and photographed using a Phenom Pro Desktop scanning electron microscope (Thermo Scientific, Waltham, MA, USA).
Nomenclature
Designation of the ventral and dorsal arms of clamp sclerites followed Bouguerche et al. [6]. Nomenclature for the male terminal genitalia of Atriaster followed Mamaev and Parukhin [45], and we considered that the male copulatory apparatus consists of 3 types of hooks: 1. genital atrium hooks: hooks on the walls of the genital atrium forming a crown; 2. paired hooks: a pair of long hooks on the muscular thickening on the anterior part of the atrium; 3. copulatory organ hooks: hooks on the muscular pad in the centre of the genital atrium. For high-level terminology of the Polyopisthocotyla, we followed the systematics of Brabec et al. [7] who recently elevated the former subclasses of “Monogenea” to the level of classes. Voucher material and type specimens were deposited in the parasite collection of the National Museum in Bloemfontein (NMB), South Africa; and the Swedish Museum of Natural History (SMNH) in Stockholm, Sweden.
Molecular methods
Generation of molecular data
DNA was extracted from the excised pieces of the worms using the PCRBiosystems Rapid DNA Extraction Kit (PCRBiosystems available from Analytical Solutions, Randburg, South Africa). The manufacturer’s protocol was followed, except only 100 μL were added during the final dilution step, instead of the recommended 900 μL, to obtain a higher concentration of DNA. The partial 28S nuclear ribosomal RNA gene and the mitochondrial cytochrome c oxidase subunit I (COI) gene were amplified by polymerase chain reaction (PCR). The 28S gene was amplified using the forward primer U178 (5′–GCA CCC GCT GAA YTT AAG–3′) and reverse primer L1642 (5′–CCA GCG CCA TCC ATT TTCA–3′) [38], following the protocol of Acosta and Smit [1]. The primer set of Asmit1 (5′–TTT TTT GGG CAT CCT GAG GTT TAT–3′) and Asmit2 (5′–TAA AGA AAG AAC ATA ATG AAA ATG–3′) [36] were used to amplify the COI gene of the Polylabris species, utilising the following protocol: denaturation at 95 °C for 15 min; followed by 35 cycles of 94 °C for 1 min, 48 °C for 2 min and 72 °C for 2 min; and a final extension of 72 °C for 10 min. Unfortunately, it was not possible to amplify the COI gene of the Atriaster species, after trials with various primer combinations and PCR conditions. PCR amplicons were visualised with 1% gel electrophoresis and sent to a commercial sequencing company (Inqaba Biotechnical Industries (Pty). Ltd., Pretoria, South Africa) for purification and sequencing. The new sequences were assembled with Geneious v. 11.1.4 bioinformatics software (Biomatters, Auckland, New Zealand). GenBank accession numbers are included in Table 1.
Sequences used in the phylogenetic analyses.
Phylogenetic analyses
Phylogenetic analyses were performed using the newly generated sequences of the microcotylids and those of closely related species available in GenBank (Table 1). Alignments for each gene region were generated using the default parameters of MUSCLE [16] as implemented in Geneious v. 7.1.3. Nucleotide substitution models for phylogenetic analyses were calculated using MEGA 7 [31]. Both 28S and COI alignments were subjected to the model GTR + I + G for phylogenetic analyses. RAxML [23] was used to generate maximum likelihood (ML) trees, estimating model parameters and bootstrap support values (1,000 repetitions). MrBayes [59] was used to generate Bayesian Inference (BI) trees, running two independent MCMC runs of four chains for 10 million generations and sampling tree topologies every 1,000 generations. Burn-in periods were set to the first 25,000 generations. Both ML and BI analyses were carried out on the computational resource CIPRES [50]. FigTree v1.4.4 was used to visualise the phylogenetic trees [57]. Genetic distance matrices were calculated using MEGA 7.
Results
Two species of microcotylids were found parasitising D. capensis. Based on morphological analyses, one species belongs to Atriaster and the other to Polylabris. Data on the prevalence and intensity of infestation for both species are presented in Table 2. The two species are regarded as new to science and are described below.
Prevalence and intensity of infection of the two microcotylid species infecting Diplodus capensis in South Africa, found in the present study (DHNR, De Hoop Nature Reserve; TNP, Tsitsikamma section of the Garden Route National Park).
Morphological characterisation
Class Polyopisthocotyla Brabec, Salomaki, Kolísko, Scholz & Kuchta, 2023
Family Microcotylidae Taschenberg, 1879
Subfamily Atriasterinae Maillard & Noisy, 1979
Genus Atriaster Lebedev & Parukhin, 1969
Atriaster ibamba n. sp. (Figs. 2–4)
urn:lsid:zoobank.org:act:96DFF280-90ED-4093-BAD5-DD1B0C9A386F
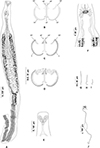 |
Figure 2 Atriaster ibamba n. sp. ex Diplodus capensis from South Africa. A, body, ventral view (NMBP1091). B, organisation of clamps sclerites in ventral jaw. C, organisation of clamps sclerites in dorsal jaw. D, clamp, ventral view (NMBP1109). E, anterior end showing rims of oral suckers (NMBP1111). F, anterior part showing male copulatory apparatus (NMBP1091). G, paired central hooks. H, outer hooks. I, inner hooks (NMBP1111). J, egg (NMBP1116). |
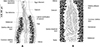 |
Figure 3 Atriaster ibamba n. sp. ex Diplodus capensis from South Africa. A, detail of anatomy in anterior body (NMBP1112). B, details of ovarian region (NMBP1114). |
 |
Figure 4 Atriaster ibamba n. sp. scanning electron micrographs [SEM]. A, anterior forebody, lateral view, parasite depositing egg; B, anterior end, ventral view; C, anterior end, arrows pointing to lateral vaginal openings; D, full body, ventral view, eggs tangled around posterior end of body; E, anterior extremity, arrows indicating apical glands; F, clamps; G, septate oral suckers; H, genital pore, solid arrow indicating paired median hooks, dotted arrow indicating lateral hooks. Abbreviations: E, egg. Scale bars: A, B, 200 μm; C, 80 μm; D, 500 μm; E, F, 50 μm; G, H, 10 μm. |
Type-host: Diplodus capensis (Smith) (Sparidae), Cape white seabream.
Type-locality: Koppie Alleen, De Hoop Nature Reserve, South Africa (34°28′42.1”S, 20°30′39.9”E).
Other localities: Witsand (34°23′49″S, 20°50′14.71”E), Mossel Bay (34°10′45.3″S, 22°09′07.4″E), the Tsitsikamma section of the Garden Route National Park (34°1′15.2112″S, 23°52′43.2264″E), and Chintsa East (32°50′11.5368″S, 28°7′1.1892″E), South Africa.
Site of infestation: gills.
Deposited examined material: Holotype (NMBP1091) and 33 paratypes (NMBP1092–NMBP1124), including four hologenophores, are deposited in the NMB; six paratypes deposited in SMNH (Type-9977–Type-9982).
Representative DNA sequences: Partial 28S rDNA, seven sequences (PV658383–PV658389).
Etymology: The species epithet ibamba, noun in apposition, is an isiXhosa word meaning “to grip”, “to hold on” or “to clamp on”. This refers to the method of attachment of this group of polyopisthocotylans.
Description
Body lanceolate (Fig. 2A), anterior part of body slender, with slight constriction at level of genital atrium in some specimens; body 3443 (1644–5481) long including haptor length, body (excluding haptor) 2264 (1181–3865) long; 371 (204–636) wide at level of testes. Haptor elongate, 1658 (315–2489) long, with 115–228 (168) clamps of “microcotylid” type, sessile, situated in two parallel rows, decreasing in size antero-posteriorly; anterior clamps 35 (22–57) long, 64 (42–109) wide; posterior clamp 29 (20–41) long, 51 (33–80) wide. Clamps formed by anterior (Fig. 2B) and posterior jaws (Fig. 2C). Ventral arm of median spring a1 Y-shaped, long, slender; distal part of a1 Y-shaped, with pointed short branches of equal size; proximal part a2 wider, T-shaped, with short branches. Dorsal arm of median spring a3 shorter than a1, distally broad. Ventral arm of ventral jaw sclerites b1, dorsal arm b2 short and curved inwards, b2 not reaching median spring. Dorsal jaw sclerites c shorter than ventral, sclerites c not reaching midline on distal side. Muscle connecting a2 and b2 present on proximal side (Fig. 2D).
Prohaptoral suckers transversely oval, 85 (60–111) long, 42 (34–51) wide, muscular, septate, divided by transverse muscular partition into two subequal chambers, lateral chamber smaller; rims of buccal suckers with rows of minute conical expansions (Fig. 2E). Three groups of apical glands, two larger lateral groups and one smaller median group in anterior extremity. Pharynx spherical to subspherical, 36 (27–51) long, 37 (28–44) wide. Oesophagus relatively long. Intestinal bifurcation anterior to genital pore; caeca with numerous short lateral ramifications, uniting posteriorly, forming diverticulum that extends along anterior portion of haptor axis.
Testes 23 (8–35) in number, postovarian, intercaecal, in posterior half of body, distributed in two rows. Copulatory apparatus consisting of three types of hooks: paired central hooks located on the muscular thickening in anterior part of genital atrium; outer hooks located on the walls of the genital atrium forming a crown; and inner hooks located on muscular pad in middle of genital atrium (Fig. 2F). Paired hooks 84 (55–105) long, rod-shaped, visibly curved ventrally (Fig. 2G). Outer hooks 16 (12–21) in number, 57 (30–72) long, thin, with distal curved ends facing interior of genital atrium (Fig. 2H). Inner hooks robust, 8 (6–10) in number, 39 (25–64) long, located in middle of the genital atrium in well mounted specimens (Fig. 2I). Genital atrium with thick muscular walls, opening ventrally as a transversely elongate pore. Vas deferens sinuous, opening into genital atrium anterodorsally (Fig. 3A).
Germarium pretesticular, intercaecal, dorsal to vitelline ducts and uterus, in form of question mark (Fig. 3B). Uterus extending anteriorly to genital atrium. Genito-intestinal duct short, projecting into right caecum. Two lateral vaginae opening between genital pore and anterior extent of vitellarium; vaginae unarmed, tegument covered with numerous small filaments in this area. Vitelline follicles dispersed in two lateral fields surrounding caeca, extending within haptor; anterior limit of vitellarium far posterior to intestinal bifurcation. Vitelline ducts Y-shaped, with two separate efferent ducts, joining in common deferent duct, ventral, at germarium level. Eggs fusiform, with bipolar filaments; anterior filament long and coiled, often coiled around next egg; posterior filament shorter, digitiform (Fig. 2J).
Remarks
Atriaster currently consists of five nominal species [70]. Atriaster ibamba n. sp. fits the diagnosis of the genus well by having a pair of longer median hooks in the genital atrium, two vaginae, and a supporting ribbed plate in the genital atrium [19, 35, 45]. This species differs from Atriaster acanthopagri Mamaev & Parukhin, 1975 by having fewer (16 vs 40) and longer (30–72 vs 41–45) outer hooks, longer (84 vs 70) paired hooks, and by having more (8 vs 5) and longer (25–64 vs 40–45) inner hooks [45]. It differs from Atriaster bifidacanthus Mamaev & Parukhin, 1975 by having fewer (12–21 vs 35–36) and shorter (30–72 vs 70–80) outer hooks, shorter paired hooks (55–105 vs 140–160), and by having fewer (6–10 vs 12–13) and smaller (25–64 vs 70–80) inner hooks [45].
Atriaster ibamba n. sp. is distinguished from Atriaster spinifer Mamaev & Parukhin, 1975 by having fewer (12–21 vs 32–35) and longer (30–72 vs 37–45) outer hooks, longer (55–105 vs 53–74) paired hooks, and by having more (6–10 vs 4–5) and longer (25–64 vs 32–40) inner hooks [45]. It differs from Atriaster maillardi Lopez-Roman & De Armas Hernandez, 1989 by having a smaller body (315–2489 × 204–636 vs 1320–5460 × 391–773), smaller clamps (22–57 × 42–109 vs 27–41 × 32–68) and fewer outer hooks (12–21 vs 20–35) [39].
One species, Atriaster heterodus Lebedev & Parukhin, 1969 was described from a locality off Walvis Bay, Namibia, within the distribution range of the host D. capensis [35]. Atriaster ibamba n. sp. differs from the former species by having more (115–228 vs 120–160) and smaller (35 × 64 vs 52 × 90) clamps, shorter outer hooks (30–72 vs 70–76), shorter paired hooks (55–105 vs 112–118), and by having fewer (6–10 vs 8–9) and shorter (25–64 vs 70–174) inner hooks.
Subfamily Prostatomicrocotylinae Yamaguti, 1968
Genus Polylabris Euzet & Cauwet, 1967
Polylabris dassie n. sp. (Figs. 5 & 6)
urn:lsid:zoobank.org:act:9636659A-D824-4EAE-9CD2-8AB7D33DAC7F
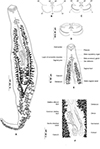 |
Figure 5 Polylabris dassie n. sp. ex Diplodus capensis from South Africa. A, body, ventral view (NMBP1125). B, organisation of clamps sclerites in ventral jaw. C, organisation of clamps sclerites in dorsal jaw. D, clamp, ventral view (NMBP1125). E, anterior end showing male copulatory organ (Type-9987). F, detail of the ovarian region. |
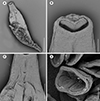 |
Figure 6 Polylabris dassie n sp. scanning electron micrographs [SEM]. A, whole body, ventral view; B, anterior extremity with oral suckers; C, genital openings; D, clamps. Scale bars: A, 500 μm; B, 50 μm; C, 100 μm; D, 20 μm. |
Type-host: Diplodus capensis (Smith) (Sparidae), Cape white seabream.
Type-locality: Chintsa East, South Africa.
Other localities: Witsand, Mossel Bay, the Tsitsikamma section of the Garden Route National Park, and Chintsa East, South Africa.
Site of infestation: gills.
Deposited examined material: Holotype (NMBP1125) and 11 paratypes (NMBP1126–NMBP1136), including four hologenophores, are deposited in the NMB; five paratypes deposited in SMNH (Type-9983–Type-9987).
Representative DNA sequences: Partial 28S rDNA, five sequences (PV627795–PV627799); Partial COI mtDNA, five sequences (PV612823–PV612827).
Etymology: This species is named after the host fish Diplodus capensis, for which the commonly used Afrikaans name in South Africa is the dassie (noun in apposition).
Description
Body lanceolate (Fig. 5A), occasionally slender, 1892–3510 (2660) long including haptor length; widest at level of ovary 376–652 (512). Haptor spatulate, pointed at anterior and posterior extremities, not well demarcated from body; bearing 64–130 (106) clamps. Clamps of “microcotylid” type, sessile, situated in two parallel rows; clamps decreasing in size towards posterior extremity of haptor; anterior clamps 24–46 (35) long, 44–98 (69) wide; posterior clamps 17–40 (26) long, 28–76 (47) wide. Clamps formed by anterior (Fig. 5B) and posterior jaws (Fig. 5C). Ventral arm of median spring a1 Y-shaped, long, slender; distal part of a1 Y-shaped, with pointed short branches of equal size; proximal part a2 wider, T-shaped, with short branches. Dorsal arm of median spring a3 shorter than a1, distally broad. Ventral arm of ventral jaw sclerites b1, dorsal arm b2 short and curved inwards, b2 not reaching median spring. Dorsal jaw sclerites c shorter than ventral, sclerites c not reaching midline on distal side. Muscle connecting a2 and b2 present on proximal side (Fig. 5D).
Prohaptoral suckers transversely oval, 79–125 (100) long, 66–97 (84) wide, muscular, divided by transverse muscular partition into two subequal chambers, lateral chamber smaller (Fig. 5E). Pharynx spherical to subspherical, 37–54 (45) long, 34–47 (39) wide. Oesophagus relatively long, with bilateral pair of diverticula. Intestinal bifurcation at level of common genital pore; caeca with numerous lateral and axial ramifications, uniting posteriorly, extends into haptor.
Testes compactly arranged in intercaecal region of middle third of body, obscured by vitellarium. Seminal vesicle opening into male copulatory organ (MCO). MCO pyriform, broader posteriorly, narrowed anteriorly, 28–40 (35) long; MCO consisting of inner tube and outer sheath; inner tube with nearly parallel margins, narrowing slightly before entering distal portion of outer sheath (Fig. 5E). Male accessory gland (MAG) ducts paired, lateral, with proximal bulbous expansion; union of ducts not examined. Genital atrium unarmed, surrounded by a circular layer of concentric muscles. Vas deferens sinuous, wide, dorsal to uterus, extending in intercaecal region anteriorly to MCO; ending in a thick-walled seminal vesicle anteriorly (Fig. 5E).
Germarium pretesticular, intercaecal, question-mark shaped, dorsal to uterus (Fig. 5F); germarium narrowing posteriorly and is followed by oviduct. Oviduct receiving genito-intestinal canal and vitello-vaginal reservoir. Genito-intestinal canal uniting with right intestinal caecum. Oviduct short, ending in an ootype. Mehlis glands not examined. Uterus thin-walled, ascending straight, ending in genital atrium. Vaginal opening ventral, posterior to pore of genital atrium (Fig. 5E). Vagina beginning with thin-walled transversal enlargement; continuing with two parallel thin-walled vaginal ducts. Vaginal ducts opening at junction of transverse vitelloducts. Vitellarium follicular, overlapping intestinal caeca, forming two lateral broad bands, uniting in posterior part of body behind testes, forming a single strip which penetrates axial part of haptor. Efferent vitelline ducts detaching in anterior third of body and uniting on midline; receiving vaginal duct anteriorly and uniting posteriorly forming vitello-vaginal ducts. Lateral vitello-vaginal ducts uniting dorsally, forming vitello-vaginal reservoir. Vitello-vaginal ducts separating into two lateral vitelline ducts posteriorly, uniting again between two transverse parts of ovary, forming common vitelline ducts. Eggs fusiform, with bipolar filaments; anterior filament long, highly coiled distally; posterior filament shorter, digitiform.
Remarks
Polylabris dassie n. sp. fits the diagnosis of this genus well, by having a sclerotised conical male copulatory organ [60]. Currently, WoRMS [71] lists 23 nominal species of Polylabris. However, according to Hayward [26], P. mamaevi Ogawa & Egusa, 1980, P. indica Hayward, 1996 and P. virgatarum (Tubangui, 1931) are species inquirenda and are thus not considered herein. WoRMS [71] lists P. longispinosus Byrnes, 1985 and P. mylionis Dillon, Hargis & Harrises, 1985 as members of Polylabris but these species were first described as Polylabroides longispinosus Byrnes, 1985 (see [11]) and Po. mylionis Dillon, Hargis & Harrises, 1985 [15], and are still maintained as valid species of Polylabroides Mamaev & Parukhin, 1976 (see Hayward [26] and the Addendum in Mamaev [44]), therefore these species are also not considered herein.
According to the number of vaginae, we followed Hayward [26] who divided the species of Polylabris into two groups: the “bivaginate group”, with paired vaginae and the “univaginate group”, with a single vaginal opening. According to Hayward [26] and subsequent descriptions of new species [60, 76], the “bivaginate group” includes six species P. australiensis Hayward, 1996; P. carnarvonensis Dillon, Hargis & Harrises, 1983; P. queenslandensis Hayward, 1996; P. sigani Dillon, Hargis & Harrises, 1983; P. sillaginae (Woolcock, 1936); and P. williamsi Hayward, 1996. The “univaginate group” includes 14 species: P. acanthogobii (Yamaguti, 1940); P. acanthopagri Mamaev & Parukhin, 1976; P. angifer Hussey, 1986; P. bengalensis Sailaja & Madhavi, 2011; P. gerres (Sandars, 1944); P. girellae Hayward, 1996; P. halichoeres Wang & Zhang, 1998; P. japonicus Ogawa & Egusa, 1980; P. kuhliae (Yamaguti, 1968); P. lingaoensis Yang, Kritsky & Pan, 2007; P. madagascariensis; P. maomao (Yamaguti, 1968); P. rhabdosargi Hayward, 1996; and P. tubicirrus (Paperna & Kohn, 1964).
By having a single mid-ventral vaginal opening, P. dassie n. sp. is classified within the “univaginate group” and is compared to other members of this group below (see also Supplementary Table S1).
Polylabris dassie n. sp. differs from P. acanthogobii by having smaller oral suckers (79–125 × 66–97 vs 36–54 × 30–48) and a smaller MCO (28–40 vs 40–45). The two species can be easily distinguished, as the tip of the MCO of P. acanthogobii has a slight constriction before widening and thickening into the dorsal recurvature, and a ventral projection; by possessing male accessory glands that are not constricted; and by having a very stout haptor, with fewer clamps [72]. This new species also differs from P. acanthopagri by having a smaller body (1892–3510 × 376–652 vs 5030–5800 × 530–560), by having fewer clamps (106 vs 160) and a shorter MCO (35 vs 50). The two species can be easily distinguished by P. dassie n. sp. lacking a thin additional process at the posterior end of the main median plate, and by sharp small teeth on the dorsal edge of the MCO. Moreover, P. dassie n. sp. is readily distinguished from P. acanthopagri by lacking the well-marked chamber extensions of vaginal ducts at some distance from the vagina (see Fig. 2b in Mamaev and Parukhin [46]).
The new species differs from P. angifer by having fewer clamps (64–130 vs 100–140) and a shorter MCO (28–40 vs 50–80), by having a straight MCO (vs armed with a sharp dorsally curved stylet at its tip in P. angifer) and by the vaginal ducts not extending laterally, close to the body margins [29]. Whereas P. dassie n. sp. can also be distinguished from P. bengalensis by having more clamps [64–130 (106) vs 64–78 (68)] and a smaller MCO [28–40 (35) vs 44–60 (54). Additionally, Polylabris dassie n. sp. is easily distinguished by having confluent vitelline fields and caeca that unite posteriorly (vs caeca ending blindly in P. bengalensis) [60].
Polylabris dassie n. sp. differs from P. gerres by having larger clamps (35 × 69 vs 25 × 58), larger oral suckers (100 × 84 vs 63) and a smaller MCO (35 vs 46) [61]. The new species is easily distinguished by having a straight MCO (vs anteriorly curved stylet at its tip in P. gerres), and by lacking the well-marked chamber extensions of the vaginal ducts (see Figure 3b in Mamaev and Parukhin [46]). Additionally, this new species differs from P. girellae by having more (64–130 (106) vs 66–78 (74)) and smaller (24–46 (35) × 44–98 (69) vs 42–51 (48) × 77–94 (88)) clamps, smaller oral suckers (79–125 (100) × 66–97 (84) vs 75–98 (86) × 24–71 (49)) and a smaller MCO (28–40 (35) vs 54–61 (58)) [26]. Polylabris dassie n. sp. can also be differentiated from P. halichoeres by having far more clamps on the haptor (64–130 vs 56–64), as well as notably larger oral suckers (79–125 × 66–97 vs 55–61 × 40–43) [79].
The new species differs from P. japonicus by having a smaller body (1892–3510 × 376–652 vs 3800–5000 × 830–930), smaller clamps (24–46 × 44–98 vs 77–91), and smaller MCO (28–40 vs 47–54). Polylabris dassie n. sp. can easily be distinguished from this species by the caeca uniting posteriorly (vs ending blindly in P. japonicus), and by lacking the chamber-like extensions of the vaginal ducts [52]. Polylabris dassie n. sp. can also be distinguished from P. kuhliae by having larger oral suckers (79–125 × 66–97 vs 37–63 × 45–63) and a smaller MCO (28–40 vs 42–50). The two species are readily distinguished by the new species lacking a styliform piece on the apex of the median spring of the clamps, and the caeca uniting posteriorly (vs ending blindly in P. kuhliae) [74].
Polylabris dassie n. sp. differs from P. lingaoensis by having a larger body (1892–3510 (2660) × 376–652 (512) vs 1130–1597 (1356) × 159–298 (236)), more numerous (64–130 vs 60–86) and larger clamps (33–40 (36) vs 44–98 (69)). Additionally, Polylabris dassie n. sp. can be easily distinguished from P. lingaoensis by possessing an MCO with a straight tip (vs dorsally recurved in P. lingaoensis) [76].
Polylabris dassie n. sp. differs from P. madagascariensis by having a larger body (1892–3510 × 376–652 vs 1350–1810 × 225–270), larger clamps (24–46 × 44–98 vs 32–34 × 59–63), double the number of clamps (64–130 vs 60–62) and a smaller MCO (28–40 vs 40–43). The two species can be easily distinguished by the tip of the MCO being strongly recurved dorsally in P. madagascariensis. Despite both species being reported from South Africa, the localities are distinct, as P. madagascariensis was first described from the sub-tropical East Coast of South Africa (Sodwana Bay) while P. dassie n. sp. is reported from the temperate South Coast of South Africa. Additionally, the host families of these two species are distinct (Sparidae vs Sillaginidae) [26].
Polylabris maomao can be distinguished from the new species by having a recurved, tapering tip of the MCO [74], whereas P. dassie n. sp. differs from P. rhabdosargi by having smaller clamps (24–46 (35) × 44–98 (69) vs 41–50 (45) × 80–91 (86)) and a smaller MCO (28–40 (35) × 41–51 (45)) [26]. Lastly, P. dassie n. sp. differs from P. tubicirrus by possessing a shorter body, slightly less clamps, larger oral suckers and a shorter MCO [54].
Molecular characterisation
28S rDNA
Partial 28S rDNA sequences were generated for seven isolates of A. ibamba n. sp. (1297–1580 bp long), and for five isolates of P. dassie n. sp. (1470–1591 bp long), parasitising D. capensis collected off the South Coast of South Africa. The newly generated sequences were compared to selected sequences of Atriasterinae, Microcotylinae and Prostatomicrocotylinae available in GenBank (Table 2; Fig. 7). Microcotyloides incisus (Linton, 1910) (MG586861) [49] was used as an outgroup. The trimmed matrix included 843 base pairs.
 |
Figure 7 Maximum likelihood phylogram based on partial sequences of the 28S rDNA gene of selected polyopisthocotylan species. Newly sequenced isolates are in bold. Posterior probability followed by bootstrap support values are given next to the branches (posterior probability < 0.90 and bootstrap < 60 not shown). Microcotyloides incisus (Linton, 1910) was used as an outgroup. Branch length scale bar indicates number of substitutions per site. |
The newly generated sequences of A. ibamba n. sp. clustered together in a strongly supported clade, and as a sister group to Atrispinum acarne Maillard & Noisy, 1979 (OL679672 and AF311702). A larger strongly supported clade was formed with isolates of Bychowskicotyla mormyri (Lorenz, 1878) (AF311713) and Sparicotyle chrysophrii (Van Beneden & Hesse, 1863) (OL679674 and AF311719), which are all part of the Atriasterinae. Figure 7 shows the phylogenetic relationships of the isolates included in the analyses of the partial 28S rDNA. The newly generated isolates of P. dassie n. sp. clustered together and as a sister group to one isolate of P. sillaginae (GU289509). A strongly supported larger clade was formed including the aforementioned isolates, one isolate of Polylabroides sp. (MH700258), one isolate of Polylabris sp. (MH700257) and Polylabris cf. mamaevi (MH700591), which are all part of the Prostamicrocotylinae. The species of the Microcotylinae, other than the one used as an outgroup, clustered together in a strongly supported clade.
The newly sequenced isolates of A. ibamba n. sp. are identical. They differ from At. acarne isolates by only 0.4–0.6% (3–4 bp). Newly sequenced isolates of P. dassie n. sp. are identical, except for isolate 18, which differed from all the other isolates by 0.1% (1 bp), which can be considered intraspecific. They differed from P. sillaginae by 2.3–2.4% (19–20 bp), and from Polylabris sp. and P. cf. mamaevi, which are identical sequences, by 2.4–2.8% (20–23 bp). Supplementary data show the values of genetic distances among all sequences included in the 28S rDNA phylogenetic analyses (Table S2).
COI mtDNAs
Partial COI mtDNA sequences were generated for five isolates of P. dassie n. sp. retrieved from D. capensis from four localities along the South African South Coast. The newly generated Polylabris COI sequences were analysed together with sequences of the Prostatomicrocotylinae and Atriasterinae available in GenBank (Table 2; Fig. 8). The plectanocotylid, Plectanocotyle gurnardi (Van Beneden & Hesse, 1863) (PP297655) [12] was selected as an outgroup. The trimmed matrix included 281 positions. The genetic code Flatworm Mitochondrial (Frame 2) was applied for translation into amino acids.
 |
Figure 8 Maximum likelihood phylogram based on sequences of the COI mtDNA gene of selected polyopisthocotylan species. Newly sequenced isolates are in bold. Posterior probability followed by bootstrap support values are given next to the branches (posterior probability < 0.90 and bootstrap < 60 not shown). Plectanocotyle gurnardi (Van Beneden & Hesse, 1863) Llewellyn, 1941 was used as an outgroup. Branch length scale bar indicates number of substitutions per site. |
The five isolates of P. dassie n. sp. clustered together in a well-supported clade, supporting the presence of a single species, P. dassie n. sp. The isolate Prostatomicrocotylinae gen. sp. (OL675212) nested within the P. dassie n. sp. clade, suggesting that it might represent the new species of Polylabris described herein. The COI tree showed the clade composed of At. acarne isolates (OL675203–OL675205) as a basal clade. A larger clade split into two lineages: one encompassing isolates of S. chrysophrii, and another encompassing Polylabris spp.
The newly generated sequences of P. dassie n. sp. are identical, as well as the sequence OL675212, suggesting their conspecificity. The isolates of P. dassie n. sp. differed by 14.6% (41 bp) from those of P. halichoeres, by 12.1% (34 bp) from P. australiensis, and by 14.6% (41 bp) from P. sillaginae. Supplementary data shows the values of genetic distances amongst all sequences included in the COI mtDNA phylogenetic analyses (Table S2).
Discussion
This is the first report of a polyopisthocotylan species from D. capensis in South Africa. The lack of knowledge about this parasitic taxon from a previously studied and widely favoured angling fish may be attributed to the scarcity of taxonomists that focus on the polyopisthocotylans of marine teleost fishes within this geographic region.
Through an integrative taxonomic approach, two species of the class Polyopisthocotyla collected from D. capensis were identified as new to science: A. ibamba n. sp. and P. dassie n. sp. This is the first report of a species of Atriaster from South Africa and we provide the first molecular sequences based on the partial 28S rDNA for microcotylids of this genus. The most recent and complete phylogeny of the Microcotylidae is that of Lablack et al. [32], in which the Atriasterinae available in GenBank were represented by Sparicotyle Mamaev, 1984, Bychowskicotyla Unnithan, 1971, and Atrispinum Euzet & Maillard, 1974. Herein, we add sequences (accompanied by permanently mounted and illustrated hologenophores) of a fourth genus, Atriaster. Despite the lack of available sequences for species of Atriaster in GenBank, the newly collected species described here as A. ibamba n. sp., corresponds well to the generic diagnosis of Atriaster by possessing a pair of longer spines at the top of the crown of spines that surround the genital atrium, two vaginae and a supporting ribbed plate in the genital atrium [35].
Currently, Atriaster includes five valid species [70], of which three were first described from the Indian Ocean (A. acanthopagri, A. bifidacanthus and A. spinifer) while the remaining were first described from the Atlantic (A. heterodus and A. maillardi). The novel species is described from the South Coast of South Africa, where the Indian and Atlantic oceans meet to create unique conditions that drive biodiversity.
Interestingly, all species of Atriaster are hosted by sparid fishes: A. bifidacanthus parasitises Sparus sp. [45], A. maillardi parasitises the zebra seabream Diplodus cervinus, A. heterodus was described from a sparid fish host [35], A. acanthopagri and A. spinifer both parasitise the twobar seabream Acanthopagrus bifasciatus [45], and A. spinifer was described from the king soldierbream Argyrops spinifer and A. bifasciatus [45]. Hence, Atriaster exhibits a stenoxenic specificity for sparid fishes. This is not unusual for Atriasterinae, for instance, all Atrispinum spp. were first described from sparid hosts: At. acarne was first described from the axillary seabream Pagellus acarne [42], Atrispinum salpae (Parona & Perugia, 1890) was first described from the Salema Sarpa salpa, and both Atrispinum sargi (Parona & Perugia, 1890) and Atrispinum seminalis (Euzet & Maillard, 1973) were both first described from the white seabream D. sargus, the common two-banded seabream D. vulgaris, and the annular seabream D. annularis [19].
A similar host specificity pattern has been examined in several polyopisthocotylans. For instance, most Gastrocotyle spp. (Gastrocotylidae) parasitise mainly Carangidae [3, 34, 64, 66]; Intracotyle spp. (Microcotylidae) are mainly known from Haemulidae [17, 33, 43, 65, 68]; Cemocotyle spp. (Heteraxinidae) are known only from Carangidae [14, 40, 47, 56]; Cotyloatlantica spp. (Chauhaneidae) and Rhinecotyle spp. (Rhinecotylidae) are known only from Sphyraena spp. [8, 20]; and Pyragraphorus spp. (Pyragraphoridae) are known only from Carangidae [18, 41, 63, 73, 78].
Polylabris is the most speciose of the Prostatomicrocotylinae and differs from other genera by its species having a sclerotised male copulatory organ. Interestingly, the newly generated COI sequences of P. dassie n. sp. are identical to those of a Prostatomicrocotylinae sp. found from D. vulgaris off Algeria, Western Mediterranean [32]. Overall, the widespread distribution of polyopisthocotylans across different regions is not uncommon and the occurrence of a single species in distinct localities has been previously verified using COI barcodes. Cappelletti and Bouguerche [12] summarised cases where the taxonomic and geographic status of several polyopisthocotylans in different localities has been validated with COI barcodes. For instance, Allogastrocotyle bivaginalis Nasir & Fuentes Zambrano, 1984 (sensu Bouguerche et al. [5]) occurs both in Mediterranean waters off Algeria [5] and in Australian waters off the southwest Pacific [28]; Kuhnia scombri (Kuhn, 1829) and Pseudokuhnia minor (Goto, 1984) were demonstrated to occur in ten localities along the coast of China [75] as well as off Australia [28]. Thus, considering the available data, the species of Prostatomicrocotylinae reported by Lablack et al. [32] is very likely conspecific with P. dassie n. sp., but morphological comparison with voucher specimens is needed to provide a definitive conclusion.
Conclusion
Atriaster ibamba n. sp. and P. dassie n. sp. are the first members of the Microcotylidae to be genetically characterised from the South Coast of South Africa, which will contribute to a better understanding of the phylogeography and delineation of this family in future studies. Considering the unique coastal habitats of South Africa, which is known to be one of the most biodiverse countries in the world, a greater diversity of these parasites would be expected. Therefore, conducting more explorative taxonomic studies on these marine parasites in this region will be highly beneficial for future work on this group.
Acknowledgments
We are grateful for the members of the North-West University (NWU) Water Research Group (WRG) for their assistance with fish collection and fieldwork. Mr. Willem Landman, NWU African Amphibian Conservation Research Group, is thanked for his invaluable assistance with the scanning electron microscopy. We would like to thank Dr Anja Erasmus (NWU-WRG) for construction of the map. This is contribution number 973 from the NWU-WRG.
Funding
This work is based on research supported in part by the Western Indian Ocean Marine Science Association (WIOMSA), Marine Research Grant I (Ref number: MARG-I-2021-CO-3 to AAA), as well as the National Research Foundation of South Africa (Ref number: MND200420515000 and PMDS23041191140 to AV). Opinions, findings, conclusions or recommendations expressed are those of the authors, and the funders accept no liability whatsoever in this regard. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Chahinez Bouguerche was supported by the Swedish Taxonomy Initiative, Artdatabanken, Swedish University of Agricultural Sciences within the scope of the project “Systematics and integrative taxonomy of Monogenea parasitizing fishes of Sweden” (SLU. 2023.4.3–248).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary materials
Table S1: Morphometric data for all valid species of Atriaster and Polylabris, including that of the new species described herein.
Table S2: Genetic divergences between species included in the phylogenetic analyses.
Access hereReferences
- Acosta AA, Smit NJ. 2021. A first for Southern Africa: description of a new Heterobothrium (Monogenea: Diclidophoridae) parasitizing the evileye pufferfish Amblyrhynchotes honckenii (Tetraodontiformes: Tetraodontidae). Parasitology Research, 120(3), 819–830. [Google Scholar]
- Badets M, Whittington I, Lalubin F, Allienne J-F, Maspimby J-L, Bentz S, Du Preez LH, Barton D, Hasegawa H, Tandon V. 2011. Correlating early evolution of parasitic platyhelminths to Gondwana breakup. Systematic Biology, 60(6), 762–781. [Google Scholar]
- Beneden HCE. 1863. Recherches sur les Bdelloïdes (Hirudinées) et les Trématodes marins. Mémoires de l’Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique, 34, 1–150 + Plates. [Google Scholar]
- Bouguerche C, Gey D, Justine J-L, Tazerouti F. 2019. Microcotyle visa n. sp. (Monogenea: Microcotylidae), a gill parasite of Pagrus caeruleostictus (Valenciennes) (Teleostei: Sparidae) off the Algerian coast, Western Mediterranean. Systematic Parasitology, 96(2), 131–147. [CrossRef] [PubMed] [Google Scholar]
- Bouguerche C, Tazerouti F, Gey D, Justine J-L. 2019. Redescription and molecular characterisation of Allogastrocotyle bivaginalis Nasir & Fuentes Zambrano, 1983 (Monogenea: Gastrocotylidae) from Trachurus picturatus (Bowdich) (Perciformes: Carangidae) off the Algerian coast, Mediterranean Sea. Systematic Parasitology, 96(8), 681–694. [Google Scholar]
- Bouguerche C, Tazerouti F, Justine J-L. 2021. Four polyopisthocotyleans (Platyhelminthes: Monogenea) from carangid fishes in the Mediterranean, off the Algerian coasts. Current Research in Parasitology & Vector-Borne Diseases, 1, 100026. [Google Scholar]
- Brabec J, Salomaki ED, Kolísko M, Scholz T, Kuchta R. 2023. The evolution of endoparasitism and complex life cycles in parasitic platyhelminths. Current Biology, 33(19), 4269–4275e3. [Google Scholar]
- Bravo-Hollis M. 1984. Monogenéa (Van Beneden, 1858) Carus, 1863 de peces marinos del litoral mexicano del Golfo de México y del mar Caribe XI. Descripción de un género y especie nuevos de la subfamilia Chauhaneinae Euzet et Trilles, 1960. Anales del Instituto de Biología. Universidad Nacional Autónoma de México, 55(2), 1–12. [Google Scholar]
- Bray RA. 1985. Some helminth parasites of marine fishes of South Africa: Families Gorgoderidae, Zoogonidae, Cephaloporidae, Acanthocolpidae and Lepocreadiidae (Digenea). Journal of Natural History, 19(2), 377–405. [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83(4), 575–583. [Google Scholar]
- Byrnes T. 1985. Four species of Polylabroides (Monogenea: Polyopisthocotylea: Microcotylidae) on Australian Bream, Acanthopagrus Spp.. Australian Journal of Zoology, 33(5), 729–742. [Google Scholar]
- Cappelletti A, Bouguerche C. 2024. “Something old, something new, something borrowed, and the oioxeny is true” : description of Plectanocotyle jeanloujustinei n. sp. (Polyopisthocotylea, Plectanocotylidae) from the MNHN Helminthology collection with novel molecular and morphological data for P. gurnardi (Van Beneden & Hesse, 1863) (sensu stricto) from Sweden. International Journal for Parasitology: Parasites and Wildlife, 23, 100914. [Google Scholar]
- Catalano SR, Hutson KS, Ratcliff RM, Whittington ID. 2010. Redescriptions of two species of microcotylid monogeneans from three arripid hosts in southern Australian waters. Systematic Parasitology, 76(3), 211–222. [Google Scholar]
- Dillon WA, Hargis WJ. 1965. Monogenetic trematodes from the Southern Pacific Ocean. 2. Polyopisthocotyleids from New Zealand fishes: the families Discocotylidae, Microcotylidae, Axinidae and Gastrocotylidae. Biology of the Antarctic Seas II, Antarctic Research Series, 5, 251–280. [Google Scholar]
- Dillon WA, Hargis WJ, Harrises AE. 1985. Monogenetic Trematodes from the southern Pacific Ocean. Polyopisthocotyleids from Australian Fishes. Subfamilies Polylabrinae (Genus Polylabroides) and Microcotylinae (Genus Neobivagina). Parazitologicheskiy Sbornik, 33, 83–87 [in Russian]. English version available from https://scholarworks.wm.edu/reports/26/. [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797. [CrossRef] [PubMed] [Google Scholar]
- Euzet L, Ktari M. 1969. Heteraxinoides hannibali n. sp. (Monogenea, Polyopisthocotylea), parasite branchial de Pomadasys incisus (Bowdich, 1825) (Teleostei) dans le Golfe de Tunis. Bulletin du Muséum National d’Histoire Naturelle, 41(1), 269–279. [Google Scholar]
- Euzet L, Ktari M. 1970. Pyragraphorus hollisae n. sp. (Monogenea) parasite of Lichia glauca (L., 1758) (Carangidae) in the Mediterranean. Anales del Instituto de Biologia Universidad Nacional Autonoma de Mexico. Zoologia, 41(1), 61–71. [Google Scholar]
- Euzet L, Maillard C. 1973. Sur deux Microcotylidae (Monogenea), parasites branchiaux de téléostéens du genre Diplodus (Sparidae). Bulletin du Muséum National d’Histoire Naturelle, 137, 793–805. [Google Scholar]
- Euzet L, Trilles J-P. 1960. Sur deux Monogènes nouveaux de Sphyraena sphyraena (L.) (Teleostei Sphyraenidae). Bulletin de la Société Zoologique de France, 85, 189–198. [Google Scholar]
- Froese R, Pauly D. 2017. FishBase. World Wide Web electronic publication. Available at: www.fishbase.org. [Google Scholar]
- Grobler NJ, van As JG, Olivier PAS. 2004. New morphological information on the parasitic copepod Caligus epinepheli Yamaguti, 1936 and Caligus rotundigenitalis Yu, 1933 (Copepoda, Caligidae) from South Africa. Crustaceana, 77(2), 187–196. [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52(5), 696–704. [CrossRef] [PubMed] [Google Scholar]
- Hadfield KA, Smit NJ, Avenant-Oldewage A. 2008. Gnathia pilosus sp. nov. (Crustacea, Isopoda, Gnathiidae) from the East Coast of South Africa. Zootaxa, 1894, 23–41. [Google Scholar]
- Hadfield KA, Bruce NL, Smit NJ. 2014. Review of the fish parasitic genus Ceratothoa Dana, 1852 (Crustacea, Isopoda, Cymothoidae) from South Africa, including the description of two new species. ZooKeys, 400, 1–42. [Google Scholar]
- Hayward CJ. 1996. Revision of the monogenean genus Polylabris (Microcotylidae). Invertebrate Taxonomy, 10(5), 995–1039. [Google Scholar]
- Heemstra PC, Heemstra E. 2004. Coastal fishes of southern Africa. Grahamstown: NISC and SAIAB, 488 p. [Google Scholar]
- Hossen MS, Barton DP, Wassens S, Shamsi S. 2022. Molecular characterisation of the monogenea parasites of blue mackerel Scomber australasicus (Perciformes: Scombridae) in Australian waters. International Journal for Parasitology: Parasites and Wildlife, 27(19), 115–127. [Google Scholar]
- Hussey CG. 1986. Some monogenean parasites of marine perciform fishes of Kuwait. Journal of Natural History, 20(2), 415–430. [Google Scholar]
- Jovelin R, Justine J-L. 2001. Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. International Journal for Parasitology, 31(4), 393–401. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547–1549. [CrossRef] [PubMed] [Google Scholar]
- Lablack L, Rima M, Georgieva S, Marzoug D, Kostadinova A. 2022. Novel molecular data for monogenean parasites of sparid fishes in the Mediterranean and a molecular phylogeny of the Microcotylidae Taschenberg, 1879. Current Research in Parasitology & Vector-Borne Diseases, 2, 100069. [Google Scholar]
- Lebedev BI. 1970. Helminths of epipelagic fishes of the South China Sea, in: Helminths of animals of Southeast Asia, Oshmarin PG, Mamaev YL, Lebedev BI, Editors. Nauka, pp. 191–208 [in Russian]. [Google Scholar]
- Lebedev BI. 1972. The taxonomy of monogeneans of suborder Gastrocotylinea. In Investigations on the fauna, systematics, and biochemistry of helminths in the far East. Proceedings of the Far-East Science Centre, USSR Academy of Sciences, 11, 121–145 [in Russian]. [Google Scholar]
- Lebedev B, Parukhin A. 1969. Monogenea of some fish from the Wallfish Bay of Southern Africa. Gidrobiologicheskii Zhurnal, 5, 70–81 [in Russian]. [Google Scholar]
- Littlewood DTJ, Rohde K, Bray RA, Herniou EA. 1999. Phylogeny of the Platyhelminthes and the evolution of parasitism. Biological Journal of the Linnean Society, 68(1–2), 257–287. [Google Scholar]
- Littlewood DTJ, Rohde K, Clough KA. 1999. The interrelationships of all major groups of Platyhelminthes: phylogenetic evidence from morphology and molecules. Biological Journal of the Linnean Society, 66(1), 75–114. [Google Scholar]
- Lockyer AE, Olson PD, Østergaard P, Rollinson D, Johnston DA, Attwood SW, Southgate VR, Horak P, Snyder SD, Le TH, Agatsuma T, McManus DP, Carmichael AC, Naem S, Littlewood DTJ. 2003. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology, 126(3), 203–224. [Google Scholar]
- López-Román R, De Armas Hernández F. 1989. Monogeneans in sea fish of Canary Archipelago. Investigation in Parasitology. Collection of Papers. Vladivostok: Vladivostok: Academy of Sciences of the USSR, Far-East Branch. p. 24–31 [in Russian]. [Google Scholar]
- MacCallum GA. 1913. Further notes on the genus Microcotyle. Zoologische Jahrbücher Abteilung für Systematik, Geographie und Biologie der Tiere, 35, 389–402. [Google Scholar]
- MacCallum GA. 1916. Some new species of parasitic trematodes of marine fishes. Zoopathologica, 1, 1–38. [Google Scholar]
- Maillard C, Noisy D. 1978. Atrispinum acarne n. g. n. sp. (Monogenea-Microcotylidae) parasite de Pagellus acarne (Teleostei) du Golfe du Lion. Vie et milieu, 29(4), 579–588. [Google Scholar]
- Mamaev YL. 1977. On one classification of monogeneans of the family Microcotylidae. Parazitologiya, 11(2), 98–103 [in Russian]. [Google Scholar]
- Mamaev YL. 1986. The taxonomical composition of the family Microcotylidae Taschenberg, 1879 (Monogenea). Folia Parasitologica, 33, 199–206. [Google Scholar]
- Mamaev YL, Parukhin A. 1975. Monogeneans of the genus Atriaster Lebedev et Parukhin, 1969 (Monogenoidea, Microcotylidae) from the Indian Ocean. Zoologicheskii Zhurnal, 54(12), 1759–1766. [Google Scholar]
- Mamaev YL, Parukhin A. 1976. Genus Polylabris Euzet et Cauwet, 1967 and some related species of microcotylids (Monogenoidea: Microcotylidae). Parazitologiia, 10(3), 245–254 [in Russian]. [Google Scholar]
- Manter HW, Prince DF. 1953. Some Monogenetic Trematodes of marine fishes from Fiji. Proceedings of the Helminthological Society of Washington, 20(2), 105–112. [Google Scholar]
- Mendoza Franco EF, Duarte Anchevida ADJ, Rosado Tun MDC. 2017. Direct Submission. Unpublished. GenBank accession number: https://www.ncbi.nlm.nih.gov/nuccore/MG586861. [Google Scholar]
- Mendoza-Franco EF, Tun MDCR, Anchevida AJD, Rodríguez REDR. 2018. Morphological and molecular (28S rRNA) data of monogeneans (Platyhelminthes) infecting the gill lamellae of marine fishes in the Campeche Bank, southwest Gulf of Mexico. ZooKeys, 783, 125–161. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for Inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 2010, 1–8. [Google Scholar]
- Mladineo I, Šegvić T, Grubišić L. 2009. Molecular evidence for the lack of transmission of the monogenean Sparicotyle chrysophrii (Monogenea, Polyopisthocotylea) and isopod Ceratothoa oestroides (Crustacea, Cymothoidae) between wild bogue (Boops boops) and cage-reared sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Aquaculture, 295(3–4), 160–167. [Google Scholar]
- Ogawa K, Egusa S. 1980. Two species of microcotylid monogeneans collected from Black Sea bream, Acanthopagrus schlegeli (Bleeker) (Teleostei: Sparidae). Japanese Journal of Parasitology, 29(6), 455–462. [Google Scholar]
- Olson PD, Littlewood DTJ. 2002. Phylogenetics of the Monogenea – evidence from a medley of molecules. International Journal for Parasitology, 32(3), 233–244. [CrossRef] [PubMed] [Google Scholar]
- Paperna I, Kohn A. 1964. Report on monogenetic trematodes collected from East Mediterranean. Revista Brasileira de Biologia, 24(3), 249–258. [Google Scholar]
- Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M. 2008. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48(1), 369–371. [CrossRef] [PubMed] [Google Scholar]
- Price EW. 1962. North American monogenetic trematodes. XI. The family Heteraxinidae. Journal of Parasitology, 48(3), 402–418. [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. 2024. FigTree v1.4.3. Available online: http://tree.bio.ed.ac.uk/software.figtree. [Google Scholar]
- Richardson TJ, Potts WM, Santos CV, Sauer WH. 2011. Ontogenetic dietary shift and morphological correlates for Diplodus capensis (Teleostei: Sparidae) in southern Angola. African Zoology, 46(2), 280–287. [Google Scholar]
- Ronquist F, Huelsenbeck JP, van der Mark P. 2005. MrBayes 3.1 Manual. Available at https://sourceforge.net/projects/mrbayes/. [Google Scholar]
- Sailaja B, Madhavi R. 2011. Polylabris bengalensis sp. nov. (Monogenea, Microcotylidae) from siganid fishes of the Visakhapatnam coast, Bay of Bengal, India. Acta Parasitologica, 56(3), 290–295. [Google Scholar]
- Sandars DF. 1944. A contribution to the knowledge of the Microcotyhlidae of Western Australia. Transactions of the Royal Society of South Australia, 68(1), 67–81. [Google Scholar]
- Smit NJ, Hadfield KA. 2015. Marine fish parasitology in South Africa: history of discovery and future direction. African Zoology, 50(2), 79–92. [Google Scholar]
- Sproston N. 1946. A synopsis of the monogenetic trematodes. Transactions of the Zoological Society of London, 25(4), 185–600. [Google Scholar]
- Subhapradha C. 1951. Vallisiopsis contorta n. g. and n. sp. and Gastrocotyle indica n. sp., monogenetic trematodes from marine fishes of the Madras coast. Parasitology, 41(3–4), 162–165. [Google Scholar]
- Tendeiro J. 1960. Sur une nouvelle espèce de trématode monogénétique, Microcotyle eduardoi n. sp. (Diclidophoroidea, Microcotylidae), parasite du Pomadasys suillus (Cuv. et Val.) (Pisces, Teleostei). Libro Homenage Jubileo (1930–1960) Doctor Eduardo Caballero, 311–314. [Google Scholar]
- Unnithan RV. 1968. On six species of monogenetic trematodes, parasitic on the gills of marine fishes from the Indian seas. Treubia, 27(2/3), 141–164. [Google Scholar]
- Vermaak A, Kudlai O, Yong RQ-Y, Smit NJ. 2023. Novel insights into the genetics, morphology, distribution and hosts of the global fish parasitic digenean Proctoeces maculatus (Looss, 1901) (Digenea: Fellodistomidae). Parasitology, 150(13), 1242–1253. [Google Scholar]
- Villalba C. 1987. Nuevas especies de monogenea en peces marinos de Chile. Parasitología al Día, 11(4), 141–148. [Google Scholar]
- Víllora-Montero M, Pérez-del-Olmo A, Georgieva S, Raga JA, Montero FE. 2020. Considerations on the taxonomy and morphology of Microcotyle spp.: redescription of M. erythrini van Beneden & Hesse, 1863 (sensu stricto) (Monogenea: Microcotylidae) and the description of a new species from Dentex dentex (L.) (Teleostei: Sparidae). Parasites & Vectors, 13(1), 45. [Google Scholar]
- WoRMS. 2024. Atriaster Lebedev & Parukhin, 1969. Accessed at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=119377 on 2024-04-25. [Google Scholar]
- WoRMS. 2024. Polylabris Euzet & Cauwet, 1967. Accessed at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=119382 on 2024-10-24. [Google Scholar]
- Yamaguti S. 1940. Studies on the helminth fauna of Japan, part 31. Trematodes of fishes. VII. Japanese Journal of Zoology, 9, 35–108. [Google Scholar]
- Yamaguti S. 1963. Systema Helminthum, Vol. IV. Monogenea and Aspidocotylea. John Wiley and Sons, Interscience Publishers: New York, 699 p. [Google Scholar]
- Yamaguti S. 1968. Monogenetic Trematodes of Hawaiian Fishes. University of Hawaii Press, Honolulu, p. 287. [Google Scholar]
- Yan S, Wang M, Yang C-P, Zhi T-T, Brown CL, Yang T-B. 2016. Comparative phylogeography of two monogenean species (Mazocraeidae) on the host of chub mackerel, Scomber japonicus, along the coast of China. Parasitology, 143(5), 594–605. [Google Scholar]
- Yang T, Kritsky DC, Pan J. 2007. Polylabris lingaoensis sp. n. and Polylabris cf. mamaevi Ogawa et Egusa, 1980 (Monogenoidea: Microcotylidae) from perciform fishes in the Gulf of Tonkin, South China Sea. Folia Parasitologica, 54(1), 27. [Google Scholar]
- Yoon G, Al-Jufaili S, Freeman M, Bron J, Paladini G, Shinn A. 2013. Omanicotyle heterospina n. gen. et n. comb. (Monogenea: Microcotylidae) from the gills of Argyrops spinifer (Forsskål) (Teleostei: Sparidae) from the Sea of Oman. Parasites & Vectors, 6(1), 170. [Google Scholar]
- Zerecero C. 1960. Pyragraphorus caballeroi n. sp. (Trematoda de la subclase Monogenea Carus, 1863) en peces marinos del Océano Pacífico del Norte, in: Libro homenaje al Dr. Eduardo Caballero y Caballero: jubileo 1930-1960, Bravo-Hollis M, Zerecero MC, Flores-Barroeta DL, Hidalgo-Escalante E, Winter HA, Editors. Instituto Politécnico Nacional: Ciudad de México. p. 345–351 (in Spanish). [Google Scholar]
- Zhang JY, Yang TB, Liu L. 2001. Monogeneans of Chinese marine fishes. Beijing: Agriculture Press, 400 pp. [Google Scholar]
- Zhang J, Wu X, Xie M, Xu X, Li A. 2011. The mitochondrial genome of Polylabris halichoeres (Monogenea: Microcotylidae). Mitochondrial DNA, 22(1–2), 3–5. [Google Scholar]
Cite this article as: Vermaak A, Bouguerche C, Acosta AA & Smit NJ. 2025. Two new species of Microcotylidae Taschenberg, 1879 (Platyhelminthes: Polyopisthocotyla) parasitising Diplodus capensis (Teleostei, Sparidae) off South Africa. Parasite 32, 44. https://doi.org/10.1051/parasite/2025037.
All Tables
Prevalence and intensity of infection of the two microcotylid species infecting Diplodus capensis in South Africa, found in the present study (DHNR, De Hoop Nature Reserve; TNP, Tsitsikamma section of the Garden Route National Park).
All Figures
 |
Figure 1 Map of sampling localities along the South African coast. Abbreviations: DHNR, De Hoop Nature Reserve; TNP, the Tsitsikamma section of the Garden Route National Park. |
| In the text | |
 |
Figure 2 Atriaster ibamba n. sp. ex Diplodus capensis from South Africa. A, body, ventral view (NMBP1091). B, organisation of clamps sclerites in ventral jaw. C, organisation of clamps sclerites in dorsal jaw. D, clamp, ventral view (NMBP1109). E, anterior end showing rims of oral suckers (NMBP1111). F, anterior part showing male copulatory apparatus (NMBP1091). G, paired central hooks. H, outer hooks. I, inner hooks (NMBP1111). J, egg (NMBP1116). |
| In the text | |
 |
Figure 3 Atriaster ibamba n. sp. ex Diplodus capensis from South Africa. A, detail of anatomy in anterior body (NMBP1112). B, details of ovarian region (NMBP1114). |
| In the text | |
 |
Figure 4 Atriaster ibamba n. sp. scanning electron micrographs [SEM]. A, anterior forebody, lateral view, parasite depositing egg; B, anterior end, ventral view; C, anterior end, arrows pointing to lateral vaginal openings; D, full body, ventral view, eggs tangled around posterior end of body; E, anterior extremity, arrows indicating apical glands; F, clamps; G, septate oral suckers; H, genital pore, solid arrow indicating paired median hooks, dotted arrow indicating lateral hooks. Abbreviations: E, egg. Scale bars: A, B, 200 μm; C, 80 μm; D, 500 μm; E, F, 50 μm; G, H, 10 μm. |
| In the text | |
 |
Figure 5 Polylabris dassie n. sp. ex Diplodus capensis from South Africa. A, body, ventral view (NMBP1125). B, organisation of clamps sclerites in ventral jaw. C, organisation of clamps sclerites in dorsal jaw. D, clamp, ventral view (NMBP1125). E, anterior end showing male copulatory organ (Type-9987). F, detail of the ovarian region. |
| In the text | |
 |
Figure 6 Polylabris dassie n sp. scanning electron micrographs [SEM]. A, whole body, ventral view; B, anterior extremity with oral suckers; C, genital openings; D, clamps. Scale bars: A, 500 μm; B, 50 μm; C, 100 μm; D, 20 μm. |
| In the text | |
 |
Figure 7 Maximum likelihood phylogram based on partial sequences of the 28S rDNA gene of selected polyopisthocotylan species. Newly sequenced isolates are in bold. Posterior probability followed by bootstrap support values are given next to the branches (posterior probability < 0.90 and bootstrap < 60 not shown). Microcotyloides incisus (Linton, 1910) was used as an outgroup. Branch length scale bar indicates number of substitutions per site. |
| In the text | |
 |
Figure 8 Maximum likelihood phylogram based on sequences of the COI mtDNA gene of selected polyopisthocotylan species. Newly sequenced isolates are in bold. Posterior probability followed by bootstrap support values are given next to the branches (posterior probability < 0.90 and bootstrap < 60 not shown). Plectanocotyle gurnardi (Van Beneden & Hesse, 1863) Llewellyn, 1941 was used as an outgroup. Branch length scale bar indicates number of substitutions per site. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


