| Issue |
Parasite
Volume 32, 2025
Special Issue – Ninth International Symposium on Monogenea. Invited Editors: Amit Tripathi, Nirupama Agarwal & Jean-Lou Justine
|
|
|---|---|---|
| Article Number | 28 | |
| Number of page(s) | 19 | |
| DOI | https://doi.org/10.1051/parasite/2025021 | |
| Published online | 12 May 2025 | |
Research Article
Monogeneans on exotic Indian freshwater fish. 7. Results of a national study on ornamental fishes from 2019–2022
Monogènes des poissons d’eau douce exotiques en Inde. 7. Résultats d’une étude nationale sur les poissons d’ornement de 2019 à 2022
1
Department of Zoology, University of Lucknow, Uttar Pradesh 226 007, India
2
Department of Veterinary and Animal Sciences, University of Copenhagen, Stigbøjlen 7, DK-1870 Frederiksberg C., Denmark
3
Department of Biology, University of Graz, Universitätsplatz 2, A-8010 Graz, Austria
* Corresponding author: tripathi_amit@lkouniv.ac.in
Received:
10
January
2025
Accepted:
4
April
2025
This study reports the results of a nationwide parasitological survey that was conducted from 2019 to 2022 to investigate the potential introduction of monogenean parasites into India via the ornamental fish trade. A total of 619 individual exotic ornamental fish representing 27 teleost species from nine families were collected from the country’s major aquaria markets and examined for monogeneans. To identify monogeneans at the species level, we employed a morphometric analysis of sclerotised structures (haptoral and reproductive hard parts), as well as a molecular analysis of nuclear 28S rRNA and ITS2 regions. Indian conditions for importing exotic ornamental fish species require a pre-quarantine certificate, quarantine treatment, and post-quarantine follow-up. Despite these restrictions, 26 monogenean species from 12 known genera were detected and identified in 17 of the 27 fishes examined. Dactylogyrus was represented by a maximum of nine species, followed by Gyrodactylus with five. Cyprinidae was the most parasitised fish family (13 species), followed by Cichlidae (three species) and Helostomatidae, Poeciliidae, and Serrasalmidae (two species each). The majority of co-transported parasite species originated from Asia (65.38%, n = 17), followed by South America (23.07%, n = 6), North and Central America (7.69%, n = 2), and Africa (3.5%, n = 1). Three fish species were identified as the first host records for monogenean parasites: Chindongo socolofi for Cichlidogyrus tilapiae Paperna, 1960, Metynnis hypsauchen for Mymarothecium sp., and Betta splendens for Heteronchocleidus sp. In general, exotic populations had fewer parasite species than in their native distribution ranges.
Résumé
Cette étude présente les résultats d’une enquête parasitologique nationale menée de 2019 à 2022 afin d’étudier l’introduction potentielle de monogènes parasites en Inde via le commerce de poissons d’ornement. Au total, 619 poissons d’ornement exotiques, représentant 27 espèces de téléostéens appartenant à neuf familles, ont été collectés sur les principaux marchés aquariophiles du pays et examinés à la recherche de Monogènes. Pour identifier les Monogènes à l’échelle de l’espèce, nous avons utilisé une analyse morphométrique des structures sclérifiées (parties dures du hapteur et de l’appareil reproducteur), ainsi qu’une analyse moléculaire des régions nucléaires de l’ARNr 28S et de l’ITS2. En Inde, les conditions d’importation des espèces de poissons d’ornement exotiques exigent un certificat de pré-quarantaine, un traitement de quarantaine et un suivi post-quarantaine. Malgré ces restrictions, 26 espèces de 12 genres connus de Monogènes ont été détectées et identifiées chez 17 des 27 poissons examinés. Dactylogyrus était représenté par un maximum de neuf espèces, suivi de Gyrodactylus avec cinq. Les Cyprinidae était la famille de poissons la plus parasitée (13 espèces), suivie des Cichlidae (trois espèces) et des Helostomatidae, Poeciliidae et Serrasalmidae (deux espèces chacune). La majorité des espèces de parasites co-transportés provenaient d’Asie (65,38 %, n = 17), suivie par l’Amérique du Sud (23,07 %, n = 6), l’Amérique du Nord et l’Amérique centrale (7,69 %, n = 2) et l’Afrique (3,5 %, n = 1). Trois espèces de poissons ont été identifiées comme premiers hôtes signalés pour des Monogènes : Chindongo socolofi pour Cichlidogyrus tilapiae Paperna, 1960, Metynnis hypsauchen pour Mymarothecium sp. et Betta splendens pour Heteronchocleidus sp. En général, les populations exotiques comptaient moins d’espèces de parasites que dans leurs aires de répartition d’origine.
Key words: Exotic ornamental fish / Monogenea / Photographic vouchers / Transboundary dissemination / 28S ribosomal RNA genes and ITS2 regions
Edited by: Jean-Loup Justine
© A. Tripathi et al., published by EDP Sciences, 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The international trade in ornamental fish, invertebrates, and plants is gaining increasing importance globally. It connects all continents through significant transfer of this specialised form of pet organism between Asia, Europe, Africa, and the Americas [30, 69, 75, 109]. Economic prospects and lucrative markets have led to impressive expansion of trade in ornamentals, which has increased from around 1 billion USD in the 1990s [4, 21] to more than 6 billion USD recently [38]. However, the import of non-endemic organisms into new geographic areas has raised concerns due to the risk of introducing potentially invasive species into vulnerable habitats, where they may outcompete local and endemic species [31]. An additional area of concern has been raised by a series of reports documenting the concomitant import of parasites with their hosts [51, 108].
India, with its relatively modest yet vibrant aquaria market, is a global hub for freshwater ornamental fishes. In 2022, the country imported ornamental fish worth 2.44 million USD, accounting for 0.72% of all global imports [73]. Though the domestic market comprises both exotic and native species of ornamental fish, exotic species are prioritised [87]. More than 170 species of ornamental fish have been introduced into India via the aquarium trade, at least 18 of which now have permanent self-reproducing populations in natural waters, especially in peninsular India [99].
As this aquaria trade facilitates the translocation of almost all known exotic aquatic parasite groups [25, 32, 58, 107], it is a perfect gateway for the translocation of monogenean parasites [99]. Monogeneans parasitise the external surfaces of fish (gills, fins, and skin), involve only one host in their life cycle, and may occur in diverse habitats, ranging from freshwater via brackish water to marine water fishes [106]. These worms feed on the blood [43] and epithelial cells and mucus of fish [17], causing direct loss due to mortality, usually to younger fish and those in intensive culture or captive conditions [95].
Exotic monogeneans could pose a serious biological invasion challenge to the fish fauna of the destination environment because (in the absence of coevolution) the native fish species lack protective immunity against exotic parasites [85, 94]. The transfer of co-introduced monogeneans of ornamental fishes to the native wild fish and the latter’s subsequent deaths is not yet well understood. Nonetheless, the global co-introductions of exotic food fish and their monogenean parasites demonstrate the devastating impact exotic monogeneans can have on the importing aquaculture industry. One example is the translocation of Gyrodactylus salaris Malmberg, 1957 from native Baltic stocks of Atlantic salmon in Sweden to Norway in the 1970s. Due to innate immunity, Baltic stocks had developed a balanced coexistence with the parasite, limiting its spread. However, Norway’s East-Atlantic salmon stocks lacked natural resistance, which allowed G. salaris to rapidly spread on these fishes [26, 57] and reduce their population in more than 51 major Norwegian rivers [66]. This resulted in economic losses exceeding 500 million USD [7]. Likewise, due to decades of intercontinental eel trading, the gill monogeneans within the genus Pseudodactylogyrus Gusev, 1965 are now prevalent on European eel in Europe, on farms and in the wild [16]. Climate change may further aggravate the risk by providing more suitable habitats for both hosts and parasites [18].
India has a rich diversity of 1,044 freshwater fishes, with 196 endemic and 822 native fishes which are economically, ecologically, and culturally important [33]. Maintaining this diversity is an important challenge for future generations, which may be jeopardised by the introduction of exotic fish and their parasites via the aquaria trade. India, as a signatory to international conventions and organisations, particularly the Convention on Biological Diversity (CBD) and the WTO agreement on Sanitary and Phytosanitary Measures (SPS Agreement), which aim to conserve biological diversity by preventing invasive alien species, has enacted the “Sanitary protocol for import of ornamental fishes into India” to effectively control and manage the introduction of ornamental fish and associated diseases into the country. The guidelines implement a twin strategy of (1) developing an “indicative list” of 97 individual species cleared for import, and (2) imposing import procedures and requirements (pre-quarantine, quarantine, and post-quarantine). These guidelines prohibit the import of any species that is not on the indicative list.
In line with pre-quarantine actions, ornamental fish cannot be imported unless accompanied by a valid import permit issued by the Government of India and a pre-quarantine certificate from the exporting countries of the consignments. Under the quarantine actions, the imported species of fish are subjected to a mandatory quarantine protocol for 15 days (21 days for goldfish) in a quarantine facility. Per the post-quarantine follow-up guidelines, it is an offence to release fish or to allow fish to escape into the wild. In addition, the guidelines prohibit the direct sale of imported brood stocks in the domestic market, allowing only the sale of F1 and F2 progeny bred in India.
Although some isolated studies have identified a limited number of exotic and invasive monogenean parasites brought into India via the ornamental trade [22, 23, 52, 98–101, 103, 104], no systematic efforts targeting Monogenea have been undertaken in the country, and very few studies have been carried out elsewhere. Therefore, this study aimed to develop a standard database on the numbers, diversity, source regions, and infection parameters (prevalence and intensity) of exotic monogenean species in India on a broad scale. This was to be accomplished using a combination of both morphological and molecular taxonomy, which would allow for the achievement of the objective in a pragmatic timeframe, while overcoming their individual drawbacks. Since the reproductive capacity of monogeneans, such as Gyrodactylus, is positively correlated to temperature [36, 66], we also evaluated the potential risk associated with their future introductions in this new era of changing climate conditions worldwide.
Materials and methods
Ethics statement
Live fishes were collected, maintained, handled, and examined in accordance with the protocol approved by the animal ethical committee of the University of Lucknow, Uttar Pradesh, India (LU/AEC/ZOO/2019 and 19/I/2024/IAEC/LU).
Study area
The sampling design employed to collect fishes included two approaches: fixed-station sampling and random sampling. For the fixed-station sampling, the domestic aquarium markets of the following five stations were selected: Delhi, Mumbai (Maharashtra), Kolkata (West Bengal), Kochi (Kerala), and Chennai (Tamil Nadu) (Fig. 1). These stations were selected for two reasons. First, they already have (or will be chosen to have) Government of India-designated seaports or airports for the import of exotic ornamental fish. Second, the fixed-station sampling design helped detect the true trends of abundance of both hosts and their parasites compared with the random sampling design. During the random sampling process, an attempt was made to collect the fishes from whichever stations possible across India, especially Uttar Pradesh, where it was possible to do so.
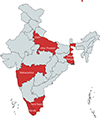 |
Figure 1 Study area map showing the collection sites (in red colour) across India (not to be scaled). |
Fish collection and examination
Between August 2019 and July 2022, 619 individuals of 27 species of ornamental fish were acquired from aquarium retailers, wholesalers, and importers. Wherever possible, live specimens were shipped to the laboratory at the University of Lucknow, UP, India on the same day they were collected after being packaged in polybags filled with water and pure oxygen. Alternatively, their gills were surgically removed and immediately fixed in hot (60 °C) 4% formalin (for morphological analysis) and 96% ethanol (for DNA analysis) before being transported. All individual fish specimens were morphologically identified using original references listed in FishBase [33]. When additional confirmation was required, the ICAR-National Bureau of Fish Genetic Resources, a leading Indian institute specialising in fish taxonomy, was consulted. Occasionally, CO1 barcoding was also utilised for identification purposes. Live fish were euthanised with an overdose of tricaine methanesulfonate (MS-222 @ 150 mg/L; Sigma Aldrich Co., St. Louis, MO, USA) until the cessation of opercular movements. Monogeneans were isolated from the gill lamellae under a stereomicroscope (Leica Microsystems, Wetzlar, Germany) using dissecting needles.
Parasite sampling
Microscopy
Formalin-fixed worms were mounted in pure glycerine, Hoyer’s medium, or 10% sodium dodecyl sulphate (SDS) [110]; a few others were stained with Gomori’s trichrome, dehydrated with a graded series of ethanol, cleared in xylene, and mounted with dibutylphthalate polystyrene xylene. Additionally, ethanol-preserved worms were treated for 20–30 min at 55 °C with 1.0 μL of digestion buffer (0.1 μL of solid tissue buffer and 0.9 μL of Proteinase-K, Zymo Quick-DNA Miniprep) to digest the tissues surrounding their haptoral and reproductive sclerotised parts.
Photographs and measurements (in micrometres) of sclerotised parts, which determine the taxonomy of monogeneans [76], were obtained using a digital camera (Leica DFC7000 T) and imaging analysis software (LAS X; Leica Microsystems) attached to a light microscope (Leica DM4B). Specimens were identified morphologically using scientific literature relevant to the group (e.g. specialist publications and monographs), and original descriptions were consulted wherever required and possible. The photographs of haptoral sclerites of all monogenean species collected are presented here to serve as photographic vouchers, allowing parasitologists worldwide to quickly compare and identify their specimens. The prevalence (percentage of infected hosts in a sample) and mean intensity (average number of parasites per infected host in a sample) of infection were determined following the methodology outlined by Bush et al. [19].
DNA extraction, amplification, and sequencing
Ethanol-preserved monogeneans were first identified morphologically before DNA extraction. Subsequently, they were subjected to centrifugation at 12,000 ×g for 1 min, followed by decanting of the preservative supernatant. The genomic DNA of individual monogeneans was extracted with either the Extracta DNA Prep for PCR-Tissue (Quantabio, Beverly, MA, USA) or Zymo Quick-DNA MiniPrep kit (Zymo Research, Irvine, CA, USA). A NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the concentration and purity of the DNA sample before polymerase chain reaction (PCR). Portions of the ribosomal gene clusters (28S and ITS2) were amplified in an automated thermal cycler (HiMedia Laboratories, Thane, MH, India) using the previously validated primers. These nuclear ribosomal DNA (rDNA) markers were chosen because they are the most commonly sequenced and versatile genes in molecular taxonomy and phylogenetics of monogenean parasites [59, 72, 74]. Additionally, the reference sequences for most of the vouchered specimens of monogeneans were available on the National Centre for Biotechnology Information (NCBI; USA) GenBank (http://www.ncbi.nlm.nih.gov/genbank/), allowing us to confidently confirm species identities via sequencing.
Primers are detailed in Table 1, while PCR reagents and their concentrations are presented in Table 2. The amplification profile for the 28S ribosomal RNA gene followed Šimková et al. [89] and the amplification profile for the ITS2 region was adapted from Hahn et al. [39] as follows: initial denaturation at 95 °C for 3 min, then 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. PCR products (2 μL) were checked for quality and length conformity on a 1.2% agarose gel pre-stained with 0.1 μL/mL of 10,000X SYBR Safe. Visualisation and documentation were done with a Bio Vision Imaging system (Vilber Lourmat, Marne-la-Vallée, France), and the most intense products were selected for sequencing. A standard 100-bp DNA ladder (HiMedia) was used to estimate the molecular weight of the amplified products.
List of primers used for amplification and sequencing in this study.
PCR reagents in the order and concentration they were added.
PCR products were purified using a 1.5% agarose gel and a QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany), then sequenced using Sanger sequencing on an ABI 3730xL automated sequencer (Applied Biosystems, Waltham, MA, USA). Sequencing was performed with the same primer combinations used for PCR amplification at the following commercial sequencing facilities in India: Barcode Biosciences, BioKart India, and Eurofins Genomics India.
Sequence quality control was performed with SnapGene version 5.3 (https://www.snapgene.com), using default parameters and manual curation of base-calling where necessary. Trimmed sequences were then assembled to produce contigs using the BioEdit Program [40], DNA Sequence Assembler v4 [28], and CAP3 assembly program [44]. Sequences were submitted to GenBank under the accession numbers indicated in Table 3.
Summary results of BLASTn search for all monogenean species identified in the current study by gene regions (28S rDNA and ITS2) (as on December 30, 2024).
Molecular species identification
The contigs were subjected to a BLAST search against the nt core database of NCBI to confirm the initial morphological identification of species. Species were identified based on a comparison of the query sequence to the subject sequences from the database with the highest index of identity, highest score, and lowest e-value.
Results and discussion
Species diversity (Table 4, Figs. 2–4)
Morphological detection
A total of 619 individual fishes belonging to 27 species, 24 genera, nine families, and seven orders were collected and examined for monogenean parasites. Of the 27 sampled host species, 17 (63%) were found to carry Monogenea. Morphological diagnoses identified 26 monogenean species. Among them, 22 were identified to the species level. Four additional records could not be identified at the species level. These may represent species new to science and will be investigated in greater depth in future work. Specifically, these species are Heteronchocleidus sp. from Betta splendens, Mymarothecium sp. from Metynnis hypsauchen, Gyrodactylus sp. from Cyprinus carpio, and Urocleidoides sp. from Xiphophorus helleri. Ten (37%) fish species were found to be free of monogenean infection. All identified species belonged to the following 12 genera: Cichlidogyrus, Dactylogyrus, Diaphorocleidus, Gussevia, Gyrodactylus, Heteronchocleidus, Heteropriapulus, Metahaliotrema, Mymarothecium, Sciadicleithrum, Trianchoratus, and Urocleidoides. Dactylogyrus had the most species with nine, followed by Gyrodactylus with five. The most parasitised fish family was Cyprinidae (13 species), followed by Cichlidae (three species) and Helostomatidae, Poeciliidae, and Serrasalmidae (two species each).
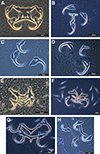 |
Figure 2 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: A) Metahaliotrema mizellei Venkatanarasaiah, 1981; B) Heteronchocleidus sp.; C) Trianchoratus acleithrium Price & Berry, 1966; D) Trianchoratus trichogasterium Lim, 1986; E) Diaphorocleidus armillatus Jogunoori, Kritsky & Venkatanarasaiah, 2004; F) Mymarothecium sp.; G) Mymarothecium viatorum Boeger, Piasecki & Sobecka, 2002; H) Gussevia asota Kritsky, Thatcher & Boeger, 1989 [Reproduced from Tripathi and Matey [104] with permission from the copyright holder]. |
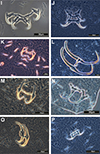 |
Figure 3 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: I) Cichlidogyrus tilapiae Paperna, 1960; J) Sciadicleithrum iphthimum Kritsky, Thatcher & Boeger, 1989; K) Dactylogyrus lampam Lim, 1992; L) Dactylogyrus anchoratus (Dujardin, 1845) Wagener, 1857; M) Dactylogyrus baueri Gussev, 1955; N) Dactylogyrus extensus Mueller & Van Cleave, 1932; O) Dactylogyrus formosus Kulwiec, 1927; P) Dactylogyrus intermedius Wegener, 1910. |
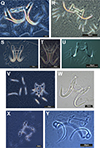 |
Figure 4 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: Q) Dactylogyrus minutus Kulwiec, 1927; R) Dactylogyrus vastator Nybelin, 1924; S) Gyrodactylus gurleyi Price 1937; T) Gyrodactylus kobayashii Hukuda, 1940; U) Gyrodactylus sp.; V) Dactylogyrus volfi Lucky, 1970; W) Gyrodactylus bullatarudis Turnbull, 1956; X) Urocleidoides sp.; Y) Heteropriapulus heterotylus (Jogunoori, Kritsky & Venkatanarasaiah, 2004) Kritsky, 2007. |
Host-parasite list indicating the exotic ornamental fish examined and monogenean parasite found (+ve indicates infection found; −ve indicates infection not found). *New host record for monogenean parasites; #First record in India.
Molecular detection (Table 3)
The PCR amplification and sequencing of the 28S rDNA or ITS2 region was successful for all 26 monogenean species recovered. Thirty-seven sequences were generated and submitted to GenBank. In most cases, molecular data supported our morphological diagnoses, with best matches generally exhibiting over 99% sequence similarity to previously deposited sequence records of the species in question, confirming the efficacy of molecular species identification.
However, two examples of the potential pitfalls of purely molecular diagnoses, especially in difficult taxonomic groups such as Monogenea, need further discussion. First, while the sequence we obtained for D. anchoratus typically confirmed our morphological species identification (multiple records of D. anchoratus with >99% similarity to ours), the BLAST search also returned a match to a record of D. formosus (PP825049.1, 100% similarity). The latter record may be a misidentification since the morphological diagnosis supporting it cannot be verified in contrast to ours (as it was deposited as a direct GenBank submission independent of publication) (see Fig. 3L), and it shows about 3% sequence divergence to other representatives of D. formosus.
Second, our sequence for T. trichogasterium (PQ249168) showed 98.78% and 97.28% similarity to records of T. acleithrium (HQ719214.1) and T. trichogasterium (HQ719217.1), respectively. As with the previous case, these latter records resulted from direct GenBank submissions and cannot be verified morphologically. Thus, we regard our record of T. trichogasterium as correct, and we consider the only other 28S record for the species representing a misidentification.
The molecular diagnosis of Dactylogyrus volfi from Puntigrus tetrazona (Bleeker, 1855) (Cyprinidae), and Urocleidoides sp. from Xiphophorus hellerii Heckel, 1848 (Poeciliidae) was challenging. Dactylogyrus volfi was first described by Lucky (1970) on Barbus tetrazona (now P. tetrazona) in Czechoslovakia. Later, Borisov (2013) recorded it from the same fish host in Bulgaria. Puntigrus tetrazona is native to Sumatra and Borneo in Indonesia (Asia), and is currently known to host only D. volfi as a monogenean parasite. Our specimens of D. volfi showed morphological consistency with Lucky’s (1970) and Borisov’s (2013) descriptions.
Although the sequencing results of all four isolates of D. volfi matched exclusively with Dactylogyrus spp. in the NCBI database, one isolate indicated a significant sequence divergence of 13.50–17.74% from the other three, which were identical. Although DNA contamination cannot be excluded as the cause of this result, the high-quality sequencing chromatogram for the deviant isolate strongly suggests a previously unrecognised cryptic species within the population of D. volfi in India, with little or no morphometric difference to distinguish them. Of note, no corresponding sequence of D. volfi is available in the NCBI nucleotide database to independently confirm the specific status of our specimens from P. tetrazona as D. volfi. Thus, genetic monitoring is crucial in detecting an overlooked cryptic monogenean species. Further verification with additional nuclear and mitochondrial gene markers is recommended.
Xiphophorus helleri, a native to tropical Mexico, hosts a single dactylogyrid species, Urocleidoides vaginoclaustrum, which Jogunoori, Kritsky, and Venkatanarasaiah (2004) described from India. BLAST analysis for the parasite recovered from X. hellerii identified a “top hit” (smallest E-value) with 85.01% similarity to Urocleidoides vanini Santos Neto & Domingues, 2023 from Erythrinus erythrinus (Bloch & Schneider, 1801) in Brazil. However, it also had a “best hit” (highest % identity) with 93.21% similarity to Onchocleidus principalis Mizelle, 1936 from Micropterus salmoides (Lacépède, 1802) in Portugal. Nevertheless, the specimens displayed all key morphological characteristics of Urocleidoides Mizelle & Price, 1964, including hook-shaped vaginal sclerite and coiled MCO with counter-clockwise rings. Of note, the NCBI database contains no reference sequence for U. vaginoclaustrum. Thus, based on similar comparative morphological characteristics, the identical original host, and the high host specificity of monogenean parasites, combined with BLAST results, we provisionally classify our specimens as Urocleidoides sp. until additional material is collected.
For the species D. armillatus, no sequence of the 28S gene was hitherto represented on GenBank. At present, the closest match to our record was D. neotropicalis Zago, Franceschini, Abdallah, Müller, Azevedo & da Silva, 2021 (MZ408906.1), with 88.30% sequence similarity. The genus Heteronchocleidus is currently represented on GenBank by only one species, H. buschkieli Bychowsky, 1957, and the record in question (AY841876.1) has 97.77% similarity to our putative Heteronchocleidus sp., validating our generic diagnosis but yielding no further insights.
Infection parameters (Table 5)
The presence of a parasite and its infection parameters in a host are the key factors in determining the parasite’s ability to enter and establish itself in the receiving country [48]. Of the 619 individuals from 27 species of exotic ornamental fish examined, 240 individuals (39%) from 17 species (63%) were confirmed to be infected with 26 species of monogenean parasites. The most parasitised fish family was Cyprinidae (13 species), followed by Cichlidae (three species) and Helostomatidae, Poeciliidae, and Serrasalmidae (two species each). Only two host species (12%) harboured multiple species infections (C. auratus and C. carpio), while 15 fishes (88%) carried one monogenean species each. The observed prevalence of monogeneans collected from certain representatives of Characiformes (G. ternetzi, P. brachypomus), Cichliformes (A. ocellatus), and Cypriniformes (B. altus, C. auratus, and C. carpio) was 100% across all the sub-regions of India where fish were collected.
Infection parameters of monogenean parasites from exotic Indian freshwater fish collected in 2019–2022. At least ten fish were examined for each species.
The cyprinid C. auratus was the fish with the highest parasite species richness, with nine taxa, followed by another cyprinid (C. carpio), with six taxa. This large symbiota (host-parasite complex) [34] of C. auratus, including seven Dactylogyrus spp. and two Gyrodactylus spp., is concerning because the concept of the pathobiome [8] suggests that diseases are caused by symbionts rather than a single principal agent. Four species of Dactylogyrus (D. anchoratus, D. extensus, D. minutus, and D. vastator) were found in two fish species (C. auratus and C. carpio), indicating that more closely related fish host species were more likely to be associated with the same parasite species. In fact, the monophyly of Dactylogyrus spp. of these two cyprinid hosts has already been suggested by Šimková et al. [88, 89] and, more recently, Tripathi et al. [103].
The high prevalence of co-translocated monogeneans in post-quarantine populations and captive-reared exotic ornamental fish species is alarming, given that they were most likely to be exposed to antiparasitic treatments in quarantine facilities at airports as well as culture ponds and in pet shops. This suggests either that monogeneans are resistant to more commonly used anti-parasitic medications or that the owners of these accomplishments are unaware of monogeneans, which prevents these worms from receiving appropriate treatment.
New host and geographic records (Table 4)
Three fish were found in the first host records for monogenean parasites ever reported: C. socolofi for C. tilapiae Paperna, 1960, M. hypsauchen for Mymarothecium sp., and B. splendens for Heteronchocleidus sp.
India provides new geographic records for the following 13 known monogeneans identified at the species level: C. tilapiae, D. baueri, D. extensus, D. formosus, D. intermedius, D. lampam, D. minutus, D. volfi, G. gurleyi, G. kobayashii, G. sprostonae, G. asota, and M. viatorum. In addition, the following three monogeneans were identified at the generic level and may represent new species: unknown spp. of Gyrodactylus, Heteronchocleidus, and Mymarothecium. Given the vast diversity of monogeneans (>7,000 known species) [37], the discovery of a new species is not surprising, especially when new hosts are examined or known hosts are examined from a new geographic locality.
Parasite lost (Table 6)
Introduced species often carry half of the number of parasite species reported in native populations; this could be due to a variety of factors, including a lower risk of parasite introduction with exotic species [97]. Our research supports this viewpoint. For instance, a comprehensive review of the literature identified four monogenean species that were described from A. ocellatus, three described from B. altus, 26 described from C. auratus, 38 described from C. carpio, four described from P. disjunctivus, P. reticulata, and S. argus each, and eight described from X. helleri. Nonetheless, we found nine monogenean species in C. auratus, six in C. carpio, and just one each in A. ocellatus, B. altus, P. disjunctivus, P. reticulata, S. argus, and X. helleri.
Similarly, several fish (Pethia conchonius, Poecilia latipinna, and Poecilia sphenops) which are known to harbour monogeneans in their native habitat, were not found to be infected in our investigation. This absence of monogeneans in our study, compared to those found naturally on their hosts throughout their native range, may be an artefact due to insufficient sampling or the real phenomenon in which they were not co-transported to India with their exotic host. However, any chance of sampling bias should have been eliminated due to the large, random, and long-term sample size of our study. Therefore, it is likely that this absence is real and that the absentee species failed to be co-transported with their host (at least until now). We suppose that these fish samples were imported into India from a specific geographic region that, by chance, was infected with only selected species (missing the boat) [60], which we were able to recover in India.
Biogeographical patterns in host-parasite associations (Tables 4 and 7, Fig. 5)
Geographical structure patterns (from local to global scales) in multispecies host-parasite assemblages explain future spill-over and spillback by parasites. However, identifying the country of origin of imported fish (and parasites) in the ornamental fish trade is challenging due to frequent trans-shipment and relabelling [6, 14]. For example, species labelled as originating from Singapore, the largest ornamental fish exporter in the world [115], are often collected from other countries [13] before being re-exported. Therefore, we indirectly evaluated the geographic location of fish (and parasites) by considering their native distribution range based on current country-level geographic borders.
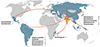 |
Figure 5 Schematic diagram representing the geographic distribution of co-introduced monogeneans recovered in India, 2019–2022. |
An overview of the native geographical regions of ornamental fish imported into India, together with their monogenean parasites.
The native distribution of collected fish from FishBase [33] indicates that the native ranges of most fish were in Asia, including the Indo–Pacific region (40.74%, n = 11), followed by South America (29.62%, n = 8), North America (7.40%, n = 2), Africa (7.40%, n = 2), North and Central America (7.40%, n = 2), South and Central America (3.70%, n = 1), and Central America (3.70%, n = 1). Concerning parasites’ specific heterogeneity, the majority of co-introduced parasite species originated from Asia (65.38%, n = 17), followed by South America (23.07%, n = 6), North and Central America (7.69%, n = 2), and Africa (3.5%, n = 1).
This distribution pattern reflects the numerical dominance of Dactylogyrus and Gyrodactylus species (nine and five, respectively) infecting cyprinid fishes from Asian countries. It also indicates that the total species richness of parasites in local host communities often correlates positively with the species richness of hosts. However, regarding generic heterogeneity, the majority of co-introduced parasite genera originated from Asia (41.66%, n = 5; Dactylogyrus, Gyrodactylus, Heteronchocleidus, Metahaliotrema, and Trianchoratus) and South America (41.66%, n = 5; Diaphorocleidus, Gussevia, Heteropriapulus, Mymarothecium, and Sciadicleithrum), followed by North and Central America (16.66%, n = 2; Gyrodactylus and Urocleidoides) and Africa (8.33%, n = 1; Cichlidogyrus).
Potentially invasive monogeneans (Table 8)
Of the 27 fish species studied, at least 10 are invasive in Indian waters. While parasite sampling of these fishes from wild waters remains to be done in the future, we collected two specimens of C. carpio and five specimens of Oreochromis niloticus (Linnaeus, 1758) from the river Ghaghra in northern India at the time of writing. However, we found only one monogenean species from each (D. minutus and Cichlidogyrus sclerosus Paperna and Thurston, 1969, respectively). This low species diversity of monogeneans may be due to the small sample size. It could also be explained by the “enemy release concept”, which holds that parasitism is substantially lower in introduced areas of invasive species than in conspecific populations in native areas [97]. Therefore, we consider the monogenean fauna of the invasive fishes detected in our study to be potential co-invaders until more extensive surveys of wild populations prove otherwise.
List of invasive Indian freshwater ornamental fish.
Additional parasites
Although the parasite sampling procedures were designed principally to detect monogenean parasites, we isolated several other metazoan parasites as well. For example, we found crustacean parasites from C. auratus. These included one copepod parasite species (Lernaea cyprinacea Linnaeus, 1758) and three brachyuran parasite species (Argulus coregoni Thorell, 1866, Argulus japonicus Thiele, 1899, and Argulus foliaceus Linnaeus, 1758). We also found some trematodes, cestodes, and nematodes but ignored them due to a lack of taxonomic expertise in these taxa. Nevertheless, given the risk of translocating parasites within these taxa between continents with uncritical ornamental fish trade [18, 64], future studies should also meticulously examine trematodes, cestodes, nematodes, crustaceans, acanthocephalans, and myxozoans. Monogeneans clearly have pathogenic potential, and because they have a single-host life cycle, they may propagate fast and colonise new hosts in new geographic regions. This, however, may not justify the exclusion of other helminth types from the regular parasitological monitoring of ornamental fish.
Illegal trafficking of ornamental fish and their parasites
Illegal wildlife trade presents many threats to public health, wildlife, and the ecosystem, serving as a primary driver of emerging infectious diseases [49]. The impact of introduced monogenean species on the native fish species is well-documented. These types of impacts have been associated with capsalid Nitzschia sturionis (Abildgaard, 1794) Krøyer, 1852 (Capsalidae), translocated from the Caspian Sea on Acipenser nudiventris Lovetsky, 1828 (Acipenseriformes: Acipenseridae) to the Aral Sea [29]; gyrodactylid Gyrodactylus salaris Malmberg, 1957 from Sweden on Salmo salar Linnaeus, 1758 (Salmoniformes: Salmonidae) to Norway [47]; and a diclidophorid Neoheterobothrium hirame Ogawa, 1999 from North America on Paralichthys olivaceus Temminck and Schlegel, 1846 (Pleuronectiformes: Paralichthyidae) to Japan [5].
Of the 27 fish species sampled, 11 (41%) were not on the government of India’s indicative list, meaning they were brought into the country through illegal trafficking. These fish are B. altus, C. socolofi, G. ternetzi, H. temminckii, H. erythrozonus, M. hypsauchen, M. ramirezi, P. brachypomus, P. sphenops, P. disjunctivus, and S. argus. Fish that have been illegally smuggled into the country present an especially great hazard because they have bypassed the import risk analysis and quarantine procedures of the importing country, and therefore, have a high probability of carrying exotic parasites. This is justified by the fact that eight of 11 (72.72%) illegally imported fishes were found to be infected with monogenean parasites, compared to nine of the 16 (56.25%) that are available on the indicative list and had passed through the quarantine process. Such illegal imports, therefore, may accelerate parasite spread, leading to disease outbreaks. As such, these fish should be added to the “indicative list” and placed in quarantine on arrival, or their sale should be considered a criminal act, comparable to wildlife smuggling, and declared punishable by severe penalties.
Risks to importing countries, especially India, with the changing global climate
Host-parasite interactions may be influenced by environmental conditions, including climate change [65], which can directly affect parasite prevalence and intensity or indirectly impact the phenology of both hosts and parasites [62]. However, predicting the consequences of climate change on host-parasite dynamics is difficult due to limited or conflicting empirical evidence available. Nonetheless, it is widely accepted that global climate change will influence the distribution of parasite diseases [3]. This could facilitate the introduction of parasites into the new environments, aggravating the already well-documented devastating consequences on endemic teleost populations following the uncritical import and release of fish [31] and their parasites [18] into natural waters.
The survival and reproduction of various parasites depend on a range of climate-related abiotic factors, such as salinity, pH, humidity, and, most notably, temperature [111]. Higher temperatures, for example, often increase parasite growth, reproduction, and infectivity [61, 92]. Therefore, fluctuations in these basic ecological factors in regions where exotic parasites have been introduced may benefit both endemic and introduced parasite species as climate conditions change. Of special note, in this context, the reproductive rates of both oviparous [15] and viviparous monogeneans [36, 56, 102] are positively correlated to temperature. Although climate change is a global concern, India is particularly vulnerable to its impacts due to its fast population growth and the general inability of its infrastructure and public services to adapt to anticipated adverse effects [86].
Conclusions and call for action
Understanding the spectrum of exotic parasites and pathogens, particularly under changing ecological and environmental conditions of intercontinental translocations, is crucial in determining the associated risks and appropriate management actions. Our study, which is the first large-scale quantitative parasitological investigation of a wide range of exotic ornamental fishes in India, established the abundance and diversity of monogenean parasites in freshwater ornamental fishes imported into the country. Morpho-molecular profiling revealed the presence of monogenean parasites in 17 of the 27 exotic ornamental fish examined. Twenty-six monogenean species were confirmed as co-introduced, nearly half of which were considered potential co-invaders. The prevalence and intensity of infection were generally high for most of the species studied, which can only be attributable to the inadequate risk assessment, detection, diagnosis, and management of quarantine systems at Indian borders.
Two key points are made in concluding this study. First, the ornamental trade is a strong driver of the transboundary translocation of monogenean parasites. Second, current biosecurity measures designed to prevent the introduction of exotic parasites into India are less stringent than previously thought, requiring alternative approaches to be explored or existing protocols to be upgraded. We hypothesise that these fishes may have similarly introduced several other protistan and metazoan parasites into India. This possibility should be investigated by taxonomists who specialize in these groups.
The data generated in this study serve three important purposes. First, the data demonstrate the efficiency of the ornamental fish trade in transporting monogenean parasites across continents. Second, the data represent essential baseline information for the prevention and management of exotic monogenean parasites in Indian aquaria and ponds. Third, and most importantly, the data raise concerns about the vulnerability of India’s quarantine barrier to the infiltration of monogenean parasites (and, potentially, other parasites).
A detailed review of the efforts made by the Government of India to protect native biodiversity from exotic species is beyond the scope of this paper. Still, it can be said that the Government of India is increasingly aware of the serious consequences of introducing exotic animals, including ornamental fishes, and their parasites. As a result, various policies have been enacted to prevent and control them. Specifically, in 2015, the Ministry of Agriculture and Farmers Welfare implemented “Guidelines for the Import of Ornamental Fishes into India” to regulate the introduction of exotic aquatic ornamental animals. In 2017, the ministry replaced these guidelines with the “Sanitary Protocol for Import of ornamental fishes into India” (see Introduction). The new “protocol” prioritises prevention of disease introduction and ensuring the health and safety of humans and the Indian aquatic environment.
Most recently, the Wildlife Protection Act of 1972, amended in 2022, added section 62A. (1), which states that “The Central Government may, by notification, regulate or prohibit the import, trade, possession or proliferation of invasive alien species which pose a threat to the wild life or habitat in India”. In 2024, the Ministry of Environment, Forest, and Climate Change updated its National Biodiversity Strategy and Action Plan, a framework used by countries to implement the Convention on Biological Diversity at the national level, establishing 23 National Biodiversity Targets, with Target 6 focussing specifically on the management of invasive alien species. Despite these seemingly strong provisions, there are clear limitations (to which this paper is a testimony); some of these were reviewed by Tripathi [99].
In this context, based on the results, we offer the following recommendations for reducing the co-transport and release of potentially harmful fish parasite species:
Separate risk analysis and quarantine provisions should be implemented for fish as pets and for the parasites they may carry.
Quarantine facilities should include highly specialised diagnostic laboratories employing a wide range of registered fish health professionals, including particularly parasite taxonomists.
Molecular-genetic identification, based on conventional and real-time PCR along with Sanger sequencing, Illumina sequencing, or their combination should be used in addition to traditional methods of identification (light microscopy) to ensure the rapid, accurate, and reliable detection and identification of parasites, especially monogeneans.
A continuous and mandatory surveillance and reporting system should be developed and enforced.
A dedicated “Central Institute of Invasive Species Management” should be established as a multidisciplinary research, teaching, and extension unit to coordinate the management of invasive species throughout India.
A dedicated and sustained funding mechanism should be developed to allow for fast responses to and the effective management of exotic fish parasites.
Funding
This study is based on the findings of a research project entitled “Morphological and molecular characterization of the parasitic monogenoids (Platyhelminthes) of exotic ornamental fish in India”, which was generously funded by the Science and Engineering Research Board (SERB), Government of India to AT (SERB–EMR/2017/003232). The results were initially presented at the “9th International Symposium on Monogenea” in October 2023 at the University of Lucknow, UP, India, and we gratefully acknowledge the participants for their constructive comments. AT is thankful to Nirupama Agarwal (University of Lucknow, India) for general discussion, and his PhD students (Nida Khwaja, Priyanka Rawat, Sneha Singh, Sneha Prakash, and Supreme Maurya) for helping with the fish collection and slide preparations. CH was supported by the Austrian Science Fund (FWF) (project P 32691).
Conflicts of interest
The authors declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Acosta AA, Franceschini L, Zago AC, Scholz T, Silva RJ. 2017. Six new species of Heteropriapulus (Monogenea: Dactylogyridae) from South American fishes with an amended diagnosis to the genus. Zootaxa, 3, 459–482. [Google Scholar]
- Ahmadi A, Borji H, Naghibi A, Nasiri MR, Sharifiyazdi H. 2017. Morphologic and molecular (28S rDNA) characterization of Dactylogyrus spp. in Cyprinus carpio and Ctenopharyngodon idella in Mashhad, Iran. Canadian Journal of Veterinary Research, 81, 280–284. [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science, 341, 514–519. [CrossRef] [PubMed] [Google Scholar]
- Andrews C. 1990. The ornamental fish trade and fish conservation. Journal of Fish Biology, 37, 57–59. [Google Scholar]
- Anshary H, Yamamoto E, Miyanaga T, Ogawa K. 2002. Infection dynamics of the monogenean Neoheterobothrium hirame infecting Japanese flounder in the western Sea of Japan. Fish Pathology, 37, 131–140. [CrossRef] [Google Scholar]
- Arthur JR, Baldock CF, Bondad-Reantaso MG, Perera R, Ponia B, Rodgers CJ. 2008. Pathogen risk analysis for biosecurity and the management of aquatic animal movements, in: Diseases in Asian aquaculture, Bondad-Reantaso MG, Mohan CV, Crumlish M, Subasinghe RP, Editors. Asian Fisheries Society: Manila. p. 21–52. [Google Scholar]
- Bakke TA, Harris PD, Hansen H, Cable J, Hansen LP. 2004. Susceptibility of Baltic and East Atlantic salmon Salmo salar stocks to Gyrodactylus salaris (Monogenea). Diseases of Aquatic Organisms, 58, 171–177. [CrossRef] [PubMed] [Google Scholar]
- Bass D, Stentiford GD, Wang H, Koskella B, Tyler CR. 2019. The pathobiome in animal and plant diseases. Trends in Ecology & Evolution, 3, 996–1008. [CrossRef] [PubMed] [Google Scholar]
- Benovics M, Kičinjaová ML, Šimková A. 2017. The phylogenetic position of enigmatic Balkan Aulopyge huegelii (Teleostei: Cyprinidae) from the perspective of host-specific Dactylogyrus parasites (Monogenea), with a description of Dactylogyrus omenti n. sp. Parasites & Vectors, 10, 547. [CrossRef] [PubMed] [Google Scholar]
- Benovics M, Desdevises Y, Vukić J, Šanda R, Šimková A. 2018. The phylogenetic relationships and species richness of host-specific Dactylogyrus parasites shaped by the biogeography of Balkan cyprinids. Scientific Reports, 8, 13006. [CrossRef] [PubMed] [Google Scholar]
- Bijukumar A. 2000. Exotic fishes and freshwater fish diversity. Zoos’ Print Journal, 15, 363–367. [CrossRef] [Google Scholar]
- Bijukumar A, Smrithy R, Sureshkumar U, George S. 2015. Invasion of South American suckermouth armoured catfishes Pterygoplichthys spp. (Loricariidae) in Kerala, India – a case study. Journal of Threatened Taxa, 7, 6987–6995. [CrossRef] [Google Scholar]
- Biondo MV, Burki RP, Aguayo F, Calado R. 2024. An updated review of the marine ornamental fish trade in the European Union. Animals, 14, 1761. [CrossRef] [PubMed] [Google Scholar]
- Biosecurity New Zealand. 2005. Import risk analysis: Ornamental Fish. Wellington: Ministry of Agriculture and Forestry. [Google Scholar]
- Buchmann K. 1988. Temperature-dependent reproduction and survival of Pseudodactylogyrus bini (Monogenea) on the European eel (Anguilla anguilla). Parasitology Research, 75, 162–164. [CrossRef] [Google Scholar]
- Buchmann K, Mellergaard S, Køie M. 1987. Pseudodactylogyrus infections in eel: a review. Diseases of Aquatic Organisms, 3, 51–57. [CrossRef] [Google Scholar]
- Buchmann K, Bresciani J. 2006. Monogenea (phylum Platyhelminthes), in Fish diseases and disorders, volume 1: protozoan and metazoan infections, 2nd edn, Woo PTK (Editors). CAB International: UK. p. 297–344. [CrossRef] [Google Scholar]
- Buchmann K, Kania PW. 2024. Transversotrema hafniensis n. sp. Infection in Poecilia reticulata by cercariae released from Melanoides tuberculata in Denmark. Acta Veterinaria Scandinavica, 66, 15. [CrossRef] [PubMed] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. International Journal of Parasitology, 83, 575–583. [CrossRef] [Google Scholar]
- Cable J, van Oosterhout C, Barson N, Harris PD. 2005. Gyrodactylus pictae n. sp. (Monogenea: Gyrodactylidae) from the Trinidadian swamp guppy Poecilia picta Regan, with a discussion on species of Gyrodactylus von Nordmann, 1832 and their poecilid hosts. Systematic Parasitology, 60, 159–164. [CrossRef] [PubMed] [Google Scholar]
- Chapman FA, Fitz-Coy SA, Thunberg EM, Adams CM. 1997. United States of America trade in ornamental fish. Journal of the World Aquaculture Society, 28, 1–10. [CrossRef] [Google Scholar]
- Chaudhary A, Verma C, Singh HS. 2016. First report on the molecular characterization of Diaphorocleidus armillatus Jogunoori et al. 2004 (Monogenea: Dactylogyridae) infecting the gills of introduced fish, Gymnocorymbus ternetzi in India. Acta Parasitologica, 61, 639–644. [CrossRef] [PubMed] [Google Scholar]
- Chaudhary A, Chiary HR, Singh HS. 2017. First molecular confirmation of the Dactylogyrus anchoratus and D. vastator (Monogenea, Dactylogyridae) from Carassius auratus in western India. BioInvasions Records, 6, 79–85. [CrossRef] [Google Scholar]
- Chinabut S, Lim LHS. 1993. Seven new species of Dactylogyrus Diesing, 1850 (Monogenea) from Puntius Hamilton (Cyprinidae) of Thailand. Raffles Bulletin of Zoology, 41, 47–59. [Google Scholar]
- Corfield J, Diggles B, Jubb C, McDowall RM, Moore A, Richards A, Rowe DK. 2007. Review of the impacts of introduced aquarium fish species that have established wild populations in Australia (Final report). Canberra: Australian Government Department of the Environment and Water Resources. [Google Scholar]
- Dalgaard MB, Nielsen CV, Buchmann K. 2003. Comparative susceptibility of two races of Salmo salar (Baltic Lule river and Atlantic Conon river strains) to infection with Gyrodactylus salaris. Diseases of Aquatic Organisms, 53, 173–176. [CrossRef] [PubMed] [Google Scholar]
- Ding XJ, Liao XH. 2005. Phylogenetic position of the monogeneans Pseudodactylogyrus, Heteronchocleidus and Trianchoratus inferred from partial 5’ terminal sequences of 28S rDNA. Acta Zootaxonomica Sinica, 30, 244–251. [Google Scholar]
- DNA Sequence Assembler v4. 2013. Heracle BioSoft. Available at https://www.dnabaser.com. [Google Scholar]
- Dogiel VA, Lutta A. 1937. Mortality among spiny sturgeon of the Aral Sea in 1936. Rybn Khoz, 12, 26–27. [Google Scholar]
- Duggan IC, Champion PD, MacIsaac HJ. 2018. Invertebrates associated with aquatic plants bought from aquarium stores in Canada and New Zealand. Biological Invasions, 20, 3167–3178. [CrossRef] [Google Scholar]
- Elvira B. 2001. Identification of non-native freshwater fishes established in Europe and assessment of their potential threats to the biological diversity, in Convention on the conservation of European wildlife and its natural habitats, Standing Committee, 21st meeting, Strasbourg, 26–30 November, Bern\T-PVS 2001\tpvs06e_2001, Council of Europe: Strasbourg. p. 1–34. [Google Scholar]
- Freyhof J, Korte E. 2005. The first record of Misgurnus anguillicaudatus in Germany. Journal of Fish Biology, 66(2), 568–571. [CrossRef] [Google Scholar]
- Froese R, Pauly D. 2024. FishBase. World Wide Web electronic publication. Available at https://www.fishbase.org (accessed on July 01, 2024). [Google Scholar]
- Galli P, Stefani F, Benzoni F, Zullini A. 2005. Introduction of alien host–parasite complexes in a natural environment and the symbiota concept. Hydrobiologia, 548, 293–299. [CrossRef] [Google Scholar]
- García-Vásquez A, Razo-Mendivil U, Rubio-Godoy M. 2015. Morphological and molecular description of eight new species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from poeciliid fishes, collected in their natural distribution range in the Gulf of Mexico slope, Mexico. Parasitology Research, 114, 3337–3355. [CrossRef] [PubMed] [Google Scholar]
- Gelnar M. 1987. Experimental verification of the effect of water temperature on micropopulation growth of Gyrodactylus katharineri Malmberg, 1964 (Monogenea) parasitizing carp fry (Cyprinus carpio L.). Folia Parasitologica, 34, 19–23. [Google Scholar]
- Gibson D, Bray R, Hunt D, Georgiev B, Scholz T, Harris P, Bakke T, Pojmanska T, Niewiadomska K, Kostadinova A, Tkach V, Bain O, Durette-Desset M, Gibbons L, Moravec F, Petter A, Dimitrova Z, Buchmann K, Valtonen E, de Jong Y. 2014. Fauna Europaea: Helminths (Animal Parasitic). Biodiversity Data Journal, 2, e1060. [CrossRef] [Google Scholar]
- Grand View Research. 2023. Ornamental fish market size, share and trends analysis report by product 2023–2030. San Francisco, USA. Available at https://www.grandviewresearch.com/industry-analysis/ornamental-fish-market. [Google Scholar]
- Hahn C, Bakke TA, Bachmann L, Weiss S, Harris PD. 2013. Morphometric and molecular characterization of Gyrodactylus teuchis Lautraite, Blanc, Thiery, Daniel & Vigneulle, 1999 (Monogenea: Gyrodactylidae) from an Austrian brown trout population. Parasitology International, 60, 480–487. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Harris P, Shinn A, Cable J, Bakke TA. 2004. Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Systematic Parasitology, 59, 1–27. [Google Scholar]
- Hassouna N, Michot B, Bachellerie JP. 1984. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Research, 12, 3563–3583. [CrossRef] [PubMed] [Google Scholar]
- Hayward CJ, Bott NJ, Naoki I, Iwashita M, Okihiro M, Nowak BF. 2007. Three species of parasites emerging on the gills of mulloway, Argyrosomus japonicus (Temminck and Schlegel, 1843), cultured in Australia. Aquaculture, 265, 27–40. [CrossRef] [Google Scholar]
- Huang X, Madan A. 1999. CAP3: A DNA sequence assembly program. Genome Research, 9, 868–877. [CrossRef] [PubMed] [Google Scholar]
- Jin X, Li W, Cheng Y, Li M, Wu S, Zou H, Wang G. 2022. Description of Gyrodactylus banmae n. sp. (Monogenea, Gyrodactylidae) parasitic on zebrafish, Danio rerio. Parasitology International, 87, 102531. [CrossRef] [PubMed] [Google Scholar]
- Jogunoori W, Kritsky D, Venkatanarasaiah J. 2004. Neotropical Monogenoidea. 46. Three new species from the gills of introduced aquarium fishes in India, the proposal of Heterotylus n. g. and Diaphorocleidus n. g., and the reassignment of some previously described species of Urocleidoides Mizelle & Price, 1964 (Polyonchoinea: Dactylogyridae). Systematic Parasitology, 58, 115–124. [CrossRef] [PubMed] [Google Scholar]
- Johnsen BO, Jensen AJ. 1991. The Gyrodacytlus story in Norway. Aquaculture, 98, 289–302. [CrossRef] [Google Scholar]
- Kahn SA, Wilson DW, Perera RP, Hayder H, Gerritty SE. 1999 . Import risk analysis on live ornamental finfish. Canberra: Australian Quarantine and Inspection Service. [Google Scholar]
- Karesh WB, Cook RA, Bennett EL, Newcomb J. 2005. Wildlife trade and global disease emergence. Emerging Infectious Disease Reports, 11, 1000–1002. [CrossRef] [PubMed] [Google Scholar]
- Kharat SS, Dahanukar N, Raut R, Mahabaleshwarkar M. 2003. Long term changes in the freshwater fish fauna in the Northern Western Ghats, Pune. Current Science, 84, 816–882. [Google Scholar]
- Kim JH, Hayward CJ, Joh SJ, Heo GJ. 2002. Parasitic infections in live freshwater tropical fishes imported to Korea. Diseases of Aquatic Organisms, 52, 169–173. [CrossRef] [PubMed] [Google Scholar]
- Km K, Chiary HR, Gazala K, Chaudhary A, Sharma B, Singh HS. 2024. First molecular identification of a non-indigenous parasite, Sciadicleithrum iphthimum Kritsky et al., 1989 (Monogenea: Dactylogyridae) in India. BioInvasions Records, 13, 267–279. [CrossRef] [Google Scholar]
- Krishnakumar K, Raghavan R, Prasad G, Bijukumar A, Sekharan M, Pereira B, Ali A. 2009. When pets become pests – exotic aquarium fishes and biological invasions in Kerala, India. Current Science, 97, 474–476. [Google Scholar]
- Kritsky DC, Thatcher VE, Boeger WA. 1989. Neotropical Monogenea. 15. Dactylogyrids from the Gills of Brazilian Cichlidae with Proposal of Sciadicleithrum gen. n. (Dactylogyridae). Proceedings of the Helminthological Society of Washington, 56, 128–140. [Google Scholar]
- Kritsky DC, Nguyen HV, Ha ND, Heckmann RA. 2016. Revision of Metahaliotrema Yamaguti, 1953 (Monogenoidea: Dactylogyridae), with new and previously described species from the spotted scat Scatophagus argus (Linnaeus) (Perciformes: Scatophagidae) in Vietnam. Systematic Parasitology, 93, 321–335. [CrossRef] [PubMed] [Google Scholar]
- Li RR, Li WX, Wu XD, Wang GT. 2014. Identification of Gyrodactylus species in goldfish (Carassius auratus) through morphological study and the analysis of the rDNA ITS sequence. Acta Hydrobiologica Sinica, 38, 903909. [Google Scholar]
- Lindenstrøm T, Sigh J, Dalgaard MB, Buchmann K. 2006. Skin expression of IL-1beta in East Atlantic salmon, Salmo salar L., highly susceptible to Gyrodactylus salaris infection is enhanced compared to a low susceptibility Baltic stock. Journal of Fish Diseases, 29, 123–128. [CrossRef] [PubMed] [Google Scholar]
- Lintermans M. 2004. Human-assisted dispersal of alien freshwater fish in Australia. New Zealand Journal of Marine and Freshwater Research, 38, 481–501. [CrossRef] [Google Scholar]
- Lockyer AE, Olson PD, Littlewood DTJ. 2003. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biological Journal of the Linnean Society, 78, 155–171. [Google Scholar]
- MacLeod CJ, Paterson AM, Tompkins DM, Duncan RP. 2010. Parasites lost – do invaders miss the boat or drown on arrival? Ecology Letters, 13, 516–527. [CrossRef] [PubMed] [Google Scholar]
- Macnab V, Barber I. 2012. Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Global Change Biology, 18, 1540–1548. [CrossRef] [Google Scholar]
- Martínez J, Merino S, Host-parasite interactions under extreme climatic conditions. Current Zoology, 57, 390–405. [Google Scholar]
- Matejusová I, Gelnar M, McBeath AJ, Collins CM, Cunningham CO. 2001. Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). International Journal for Parasitology, 31, 738–745. [CrossRef] [PubMed] [Google Scholar]
- Mehrdana F, Jensen HM, Kania PW, Buchmann K. 2014. Import of exotic and zoonotic trematodes (Heterophyidae: Centrocestus sp.) in Xiphophorus maculatus: Implications for ornamental fish import control in Europe. Acta Parasitologica, 59, 276–283. [PubMed] [Google Scholar]
- Merino S, Møller AP. 2010. Host-parasite interactions and climate change, in Effects of climate change on birds, Møller AP, Fiedler W, Berthold P, (Editors). Oxford University Press: Oxford. p. 213–226. [Google Scholar]
- Mo TA. 2020. Gyrodactylosis (Gyrodactylus salaris), in Climate change and Infectious fish diseases,Woo PTK, Leong JA, Buchmann K, Editors, CABI: UK. p. 404–422. [Google Scholar]
- Molokomme PS, Benovics M, Luus-Powell WJ, Lukhele LP, Přikrylová I. 2023. Dactylogyrus spp. (Dactylogyridae, Monogenea) from tinfoil barb, Barbonymus schwanenfeldii imported into South Africa: morphometric and molecular characterisation. Parasite, 30, 29. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Moreira J, Luque JL, Šimková A. 2019. The phylogenetic position of Anacanthorus (Monogenea, Dactylogyridae) parasitizing Brazilian serrasalmids (Characiformes). Parasite, 26, 44. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Ng TH, Tan SK, Wong WH, Meier R, Chan S-Y, Tan HH, Yeo DCJ. 2016. Molluscs for sale: Assessment of freshwater gastropods and bivalves in the ornamental pet trade. PLoS One, 11, e0161130. [CrossRef] [PubMed] [Google Scholar]
- Nitta M, Nagasawa K. 2016. Four alien monogeneans, Including Trinigyrus peregrinus n. sp., parasitic on the invasive armored catfish Pterygoplichthys disjunctivus (Siluriformes: Loricariidae) from Okinawa-jima Island, Okinawa Prefecture, Japan. Species Diversity, 21, 95–104. [CrossRef] [Google Scholar]
- Nitta M. 2023. New records of monogeneans, Gyrodactylus cyprini (Gyrodactylidae) and Dactylogyrus extensus (Dactylogyridae), parasitic on reared common carp Cyprinus carpio (Cypriniformes: Cyprinidae) in Mie Prefecture, Japan. Species Diversity, 28, 273–284. [CrossRef] [Google Scholar]
- Nolan MJ, Cribb TH. 2005. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Advances in Parasitology, 60, 101–163. [CrossRef] [PubMed] [Google Scholar]
- OEC. 2022. Ornamental fish. Available at https://oec.world/en/profile/hs/ornamental-fish (accessed on July 01, 2024). [Google Scholar]
- Olson PD, DTJ Littlewood. 2002. Phylogenetics of the Monogenea – evidence from a medley of molecules. International Journal of Parasitology, 32, 233–244. [CrossRef] [Google Scholar]
- Perales Macedo DMB, Diaz Pernett SC, Diaz González MG, Torres Nieves GM, Santos Flores CJ, Diaz Lameiro AM, Locke SA. 2022. Autochthonous transmission of the Indomalayan parasite, Transversotrema patialense, in the Caribbean: Molecular, morphological, and experimental evidence. Experimental Parasitology, 242, 108368. [CrossRef] [PubMed] [Google Scholar]
- Pugachev ON, Gerasev PI, Gussev AV, Ergens R, Khotenowsky I. 2010 . Guide to Monogenoidea of freshwater fish of Palaearctic and Amur Regions. Milan: Ledizione-Ledi Publishing. [Google Scholar]
- Raghavan R, Prasad G, Anvar-Ali PH, Pereira B. 2008. Exotic fish species in a global biodiversity hotspot: observations from River Chalakudy, part of Western Ghats, Kerala, India. Biological Invasions, 10, 37–40. [CrossRef] [Google Scholar]
- Raghavan R, Prasad G, Anvar-Ali PH, Pereira B. 2008. Fish fauna of Chalakudy River, part of Western Ghats biodiversity hotspot, Kerala, India: patterns of distribution, threats and conservation needs. Biodiversity Conservation, 17, 3119–3131. [CrossRef] [Google Scholar]
- Řehulková E, Seifertová M, Francová K, Šimková A. 2023. Nearctic Dactylogyrus species (Platyhelminthes, Monogenea) parasitizing cypriniform fishes in the context of morphology and phylogeny, with descriptions of seven new species. Parasite, 30, 30. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Rema Devi K. 1987. A golden variation. Black Buck, 3, 22–24. [Google Scholar]
- Rema Devi K, Indra TJ, Emiliyamma KG. 1996. On the fish collections from Kerala, deposited in the Southern Regional Station, Zoological Survey of India by NRM, Stockholm. Records of the Zoological Survey of India, 95, 129–146. [CrossRef] [Google Scholar]
- Roohi DJ, Dalimi A, Pourkazemi MA, Shamsi S. 2019. Occurrence of dactylogyrid and gyrodactylid Monogenea on common carp, Cyprinus carpio, in the Southern Caspian Sea Basin. Parasitology International, 73, 1–6. [Google Scholar]
- Roshith CM, Sharma AP, Manna RK, Satpathy BB, Bhaumik U. 2013. Ichthyofaunal diversity, assemblage structure and seasonal dynamics in the freshwater tidal stretch of Hooghly estuary along the Gangetic delta. Aquatic Ecosystem Health & Management, 16, 445–453. [CrossRef] [Google Scholar]
- Santos Neto JF, Domingues MV. 2023. Integrative taxonomy of Urocleidoides spp. (Monogenoidea: Dactylogyridae) parasites of characiform and gymnotiform fishes from the coastal drainages of the Eastern Amazon, Brazil. Journal of Helminthology, 97, e64. [CrossRef] [PubMed] [Google Scholar]
- Schmid-Hempel P. 2011. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford: Oxford University Press. [Google Scholar]
- Sharma D, Tomar S. 2010. Mainstreaming climate change adaptation in Indian cities. Environment and Urbanization, 22, 451–465. [CrossRef] [Google Scholar]
- Silas EG, Gopalakrishnan A, Ramachandran A, Anna Mercy TV, Sarkar K, Pushpangadan KR, Anil KP, Ram Mohan MK, Anikuttan KK. 2011. Guidelines for green certification of freshwater ornamental fish. Kochi: The Marine Products Export Development Authority. [Google Scholar]
- Šimková A, Morand S, Jobet E, Gelnar M, Verneau O. 2004. Molecular phylogeny of congeneric monogenean parasites (Dactylogyrus): a case of intrahost speciation. Evolution, 58, 1001–1018. [PubMed] [Google Scholar]
- Šimková A, Matejsova I, Cunningham C. 2006. A molecular phylogeny of the Dactylogyridae sensu Kritsky and Boeger, 1989 (Monogenea) based on the D1–D3 domains of large subunit rDNA. Parasitology, 133, 43–53. [CrossRef] [PubMed] [Google Scholar]
- Šimková A, Řehulková E, Rasoloariniaina JR, Jorissen MWP, Scholz T, Faltýnková A, Mašová S, Vanhove MPM. 2019. Transmission of parasites from introduced tilapias: a new threat to endemic Malagasy ichthyofauna. Biological Invasions, 21, 803–819. [CrossRef] [Google Scholar]
- Singh AK, Pathak AK, Lakra WS. 2010. Invasion of an exotic fish – common carp, Cyprinus carpio L. (Actinopterygii: Cypriniformes: Cyprinidae) in the Ganga River, India and its impacts. Acta Ichthyologica et Piscatoria, 40, 11–19. [CrossRef] [Google Scholar]
- Studer A, Thieltges D, Poulin R. 2010. Parasites and global warming: net effects of temperature on an intertidal host–parasite system. Marine Ecology Progress Series, 415, 11–22. [CrossRef] [Google Scholar]
- Suresh VR, Ekka A, Biswas DK, Sahu SK, Yousuf A, Das S. 2019. Vermiculated sailfin catfish, Pterygoplichthys disjunctivus (Actinopterygii Siluriformes: Loricariidae): Invasion, biology, and initial impacts in east Kolkata Wetlands, India. Acta Ichthyologica et Piscatoria, 49, 221–233. [CrossRef] [Google Scholar]
- Taraschewski H. 2006. Hosts and parasites as aliens. Journal of Helminthology, 80, 99–128. [CrossRef] [PubMed] [Google Scholar]
- Thoney DA, Hargis Jr WH. 1991. Monogenea (Platyhelminthes) as hazards for fish in confinement. Annual Review of Fish Diseases, 1, 133–153. [CrossRef] [Google Scholar]
- Tiknaik A, Kalyankar A, Shingare M, Suryawanshi R, Prakash B, Sontakke TA, Nalage D, Sanil R, Khedkar G. 2019. Refutation of media reports on introduction of the red bellied piranha and potential impacts on aquatic biodiversity in India. Mitochondrial DNA Part A, 30, 643–650. [CrossRef] [PubMed] [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. 2003. Introduced species and their missing parasites. Nature, 421, 628–630. [CrossRef] [PubMed] [Google Scholar]
- Tripathi A, Agrawal N, Srivastava N. 2010. Monogenoidea on exotic Indian freshwater fishes. 1. A new geographical record of Sciadicleithrum iphthimum Kritsky, Thatcher, and Boeger, 1989 (Dactylogyridae) with the first description of its egg. Comparative Parasitology, 77, 83–86. [CrossRef] [Google Scholar]
- Tripathi A. 2014. The invasive potential of parasitic monogenoids (Platyhelminthes) via the aquarium fish trade: an appraisal with special reference to India. Reviews in Aquaculture, 6, 147–161. [CrossRef] [Google Scholar]
- Tripathi A, Rajvanshi S, Agrawal N. 2014. Monogenoidea on exotic Indian freshwater fishes. 2. Range expansion of Thaparocleidus caecus and T. siamensis (Dactylogyridae) by introduction of striped catfish Pangasianodon hypophthalmus (Pangasiidae). Helminthologia, 51, 23–30. [CrossRef] [Google Scholar]
- Tripathi A. 2015. Monogenoidea on exotic Indian freshwater fish. 3. Are Indian guidelines for importation of exotic aquarium fish useful and can they be implemented; the case of Neotropical Gussevia spiralocirra Kohn and Paperna, 1964. Current Science, 108, 2101–2105. [Google Scholar]
- Tripathi A, Trivedi AK, Agrawal N. 2021. Some aspects of seasonal dynamics of Dactylogyroides tripathii (Platyhelminths: Monogenoidea) parasitising pool barb Puntius sophore (Actinopteri: Cyprinidae) in India. Scientia Parasitologica, 22, 79–87. [Google Scholar]
- Tripathi A, Matey C, Agarwal N. 2022. Monogenoidea on exotic Indian freshwater fish. 4. Dactylogyrus minutus from platinum ogon, an ornamental variety of the common carp Cyprinus carpio (Cypriniformes, Cyprinidae). BioInvasions Record, 11, 510–523. [CrossRef] [Google Scholar]
- Tripathi A, Matey C. 2023. Monogenea on exotic Indian freshwater fish. 5. First report of pathogenic Gussevia asota (Platyhelminths) from oscar Astronotus ocellatus (Agassiz 1831) (Perciformes: Cichlidae). Zootaxa, 5231, 52–64. [CrossRef] [PubMed] [Google Scholar]
- Trujillo-González A, Becker JA, Vaughan DB, Huston KS. 2018. Monogenean parasites infect ornamental fish imported to Australia. Parasitology Research, 117, 995–1011. [CrossRef] [PubMed] [Google Scholar]
- Whittington ID, Cribb BW, Hamwood TE, Halliday JA. 2000. Host-specificity of monogenean (platyhelminth) parasites: a role for anterior adhesive areas? International Journal for Parasitology, 30, 305–320. [CrossRef] [PubMed] [Google Scholar]
- Whittington R, Chong R. 2007. Global trade in ornamental fish from an Australian perspective: the case for revised import risk analysis and management strategies. Preventive Veterinary Medicine, 81, 92–116. [CrossRef] [PubMed] [Google Scholar]
- Wilson JR, Saunders RJ, Hutson KS. 2019. Parasites of the invasive tilapia Oreochromis mossambicus: evidence for co-introduction. Aquatic Invasions, 14, 332–349. [CrossRef] [Google Scholar]
- Womble MR, Cox-Gardiner SJ, Cribb TH, Bullard SA. 2015. First record of Transversotrema Witenberg, 1944 (Digenea) from the Americas, with comments on the taxonomy of Transversotrema patialense (Soparkar, 1924) Cruz and Sathananthan, 1960 and an updated list of its hosts and geographic distribution. Journal of Parasitology, 101, 717–725. [CrossRef] [PubMed] [Google Scholar]
- Wong WL, Tan WB, Lim LHS. 2006. Sodium dodecyl sulphate as a rapid clearing agent for studying the hard parts of monogeneans and nematodes. Journal of Helminthology, 80, 87–90. [CrossRef] [PubMed] [Google Scholar]
- Woo PTK, Leong JA, Buchmann K. 2020. Climate change and infectious fish diseases. UK: CABI. [Google Scholar]
- Wu XY, Li AX, Zhu XQ, Xie MQ. 2005. Description of Pseudorhabdosynochus seabassi sp. n. (Monogenea: Diplectanidae) from Lates calcarifer and revision of the phylogenetic position of Diplectanum grouperi (Monogenea: Diplectanidae) based on rDNA sequence data. Folia Parasitologica, 52, 231. [CrossRef] [PubMed] [Google Scholar]
- Wu XY, Zhu XQ, Xie MQ, Li AX. 2006. The radiation of Haliotrema (Monogenea: Dactylogyridae: Ancyrocephalinae): molecular evidence and explanation inferred from LSU rDNA sequences. Parasitology, 132, 659–668. [PubMed] [Google Scholar]
- Xie Z, Ma J, Yang K, Duan C, Guo A, Yue C. 2019. Morphological description and molecular phylogeny of the Gussevia asota parasite on Astronotus ocellatus. Progress in Fishery Sciences, 40, 87–93. [Google Scholar]
- Yue G. 2019. The ornamental fish industry in Singapore. Journal of Fisheries of China, 43, 116–127. [Google Scholar]
- Zago AC, Franceschini L, Abdallah VD, Muller MI, Azevedo RK, da Silva RJ. 2021. Morphological and molecular data of new species of Characithecium and Diaphorocleidus (Monogenea: Dactylogyridae) from Neotropical characid fishes. Parasitology International, 84, 102406. [CrossRef] [PubMed] [Google Scholar]
- Ziętara MS, Lumme J. 2004. Comparison of molecular phylogeny and morphological systematics in fish parasite genus Gyrodactylus Nordmann, 1832 (Monogenea, Gyrodactylidae). Zoologica Poloniae, 49, 5–28. [Google Scholar]
Cite this article as: Tripathi A, Matey C, Buchmann K & Hahn C. 2025. Monogeneans on exotic Indian freshwater fish. 7. Results of a national study on ornamental fishes from 2019–2022. Parasite 32, 28. https://doi.org/10.1051/parasite/2025021.
All Tables
Summary results of BLASTn search for all monogenean species identified in the current study by gene regions (28S rDNA and ITS2) (as on December 30, 2024).
Host-parasite list indicating the exotic ornamental fish examined and monogenean parasite found (+ve indicates infection found; −ve indicates infection not found). *New host record for monogenean parasites; #First record in India.
Infection parameters of monogenean parasites from exotic Indian freshwater fish collected in 2019–2022. At least ten fish were examined for each species.
An overview of the native geographical regions of ornamental fish imported into India, together with their monogenean parasites.
All Figures
 |
Figure 1 Study area map showing the collection sites (in red colour) across India (not to be scaled). |
| In the text | |
 |
Figure 2 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: A) Metahaliotrema mizellei Venkatanarasaiah, 1981; B) Heteronchocleidus sp.; C) Trianchoratus acleithrium Price & Berry, 1966; D) Trianchoratus trichogasterium Lim, 1986; E) Diaphorocleidus armillatus Jogunoori, Kritsky & Venkatanarasaiah, 2004; F) Mymarothecium sp.; G) Mymarothecium viatorum Boeger, Piasecki & Sobecka, 2002; H) Gussevia asota Kritsky, Thatcher & Boeger, 1989 [Reproduced from Tripathi and Matey [104] with permission from the copyright holder]. |
| In the text | |
 |
Figure 3 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: I) Cichlidogyrus tilapiae Paperna, 1960; J) Sciadicleithrum iphthimum Kritsky, Thatcher & Boeger, 1989; K) Dactylogyrus lampam Lim, 1992; L) Dactylogyrus anchoratus (Dujardin, 1845) Wagener, 1857; M) Dactylogyrus baueri Gussev, 1955; N) Dactylogyrus extensus Mueller & Van Cleave, 1932; O) Dactylogyrus formosus Kulwiec, 1927; P) Dactylogyrus intermedius Wegener, 1910. |
| In the text | |
 |
Figure 4 Phase-contrast images of haptoral armaments (anchor-bar complex and hooks) of monogenean species: Q) Dactylogyrus minutus Kulwiec, 1927; R) Dactylogyrus vastator Nybelin, 1924; S) Gyrodactylus gurleyi Price 1937; T) Gyrodactylus kobayashii Hukuda, 1940; U) Gyrodactylus sp.; V) Dactylogyrus volfi Lucky, 1970; W) Gyrodactylus bullatarudis Turnbull, 1956; X) Urocleidoides sp.; Y) Heteropriapulus heterotylus (Jogunoori, Kritsky & Venkatanarasaiah, 2004) Kritsky, 2007. |
| In the text | |
 |
Figure 5 Schematic diagram representing the geographic distribution of co-introduced monogeneans recovered in India, 2019–2022. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


