This article is a note for:
[https://doi.org/10.1051/parasite/2018028]
| Issue |
Parasite
Volume 25, 2018
|
|
|---|---|---|
| Article Number | 31 | |
| Number of page(s) | 11 | |
| DOI | https://doi.org/10.1051/parasite/2018029 | |
| Published online | 28 May 2018 | |
Research Article
Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas
Part 2. Distinct canine and feline host association with two different Dipylidium caninum genotypes
Analyse des ténias Dipylidium caninum des chiens et des chats, ou de leurs puces respectives
Partie 2. Association distincte des hôtes canins et félins avec deux génotypes différents de Dipylidium caninum
1
Boehringer Ingelheim Animal Health,
29 Av T. Garnier,
69007
Lyon, France
2
Clinomics, P.O. Box 11186, Universitas,
Bloemfontein,
9321, South Africa
3
Clinvet, P.O. Box 11186, Universitas,
Bloemfontein,
9321, South Africa
* Corresponding author: frederic.beugnet@merial.com
Received:
10
January
2018
Accepted:
21
April
2018
Initial investigations suggested the existence of two distinct genotypes of Dipylidium caninum from infected cat fleas (Ctenocephalides felis). One genotype was found almost always (> 95%) in fleas collected from, and proglottids shed by, domestic dogs. The other was found almost always (> 95%) in fleas collected from, and proglottids shed by, domestic cats. Molecular investigations (Part 1, in this journal) confirmed the presence of two distinct genotypes. Due to the apparent host association observed, these were referred to as the “D. caninum canine genotype” and the “D. caninum feline genotype”. The current article reports on an in vivo experimental infection study assessing the host-parasite interaction for each genotype. Mixed infections with the two genotypes in both dogs and cats were conducted. The specific genotyping of proglottids allowed us to assess the specific prepatent periods, prolificity, and longevity of each genotype in dogs versus cats. The possible hybridisation was also studied through molecular evaluation of the proglottids expelled by infected dogs and cats. Results demonstrate a clear distinct host interaction. The canine D. caninum genotype occurred at a higher frequency in dogs, with a shorter prepatent period and a longer lifespan; and the feline genotype occurred at a higher frequency in cats, with a shorter prepatent period and a longer lifespan. The absence of any hybrids in the mixed infections of both dogs and cats confirm the hypothesis of two distinct genotypes, suggesting the possibility of two distinct species within Dipylidium caninum.
Résumé
Des investigations initiales ont suggéré l’existence de deux génotypes distincts au sein de Dipylidium caninum issus de puces infectées (Ctenocephalides felis). Un génotype est trouvé dans plus de 95 % des cas chez des puces ou des proglottis collectés sur des chiens. L’autre est trouvé dans plus de 95 % des cas sur des puces collectées sur des chats ou des proglottis éliminés par les chats. Les investigations moléculaires publiées (Partie 1, dans ce journal) ont confirmé l’existence de ces deux génotypes. Du fait de l’apparent tropisme d’hôte, ces deux génotypes sont désignés comme génotype canin et génotype félin. Le présent article présente les résultats d’infestations expérimentales ayant pour objectif d’étudier l’interaction hôte-parasite pour chaque génotype. Des infestations mixtes ont été réalisées avec les deux génotypes chez des chiens et des chats. Le génotypage spécifique a permis d’étudier les périodes prépatentes, la prolificité et la longévité de chaque génotype chez chaque hôte. La possible hybridation a aussi été étudiée par évaluation moléculaire des proglottis éliminés par les chiens et les chats infestés. Les résultats ont démontré une interaction hôte-parasite bien distincte. Le génotype canin de D. caninum a une fréquence plus élevée chez les chiens, avec une période de prépatence plus courte et une durée de vie plus longue, et le génotype félin a une fréquence plus élevée chez les chats, avec une période prépatente plus courte et une durée de vie plus longue. L’absence de tout hybride dans les infections mixtes des chiens et des chats confirme l’hypothèse de deux génotypes distincts, suggérant la possibilité de deux espèces distinctes au sein de Dipylidium caninum.
Key words: Dipylidium caninum / Ctenocephalides felis / dogs / cats / genotypes / host association
© F. Beugnet et al., published by EDP Sciences, 2018
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Dipylidium caninum sensu lato is an important cestode parasite with a worldwide distribution, as is evident from surveys performed in wild canids and felids, domestic cats, domestic dogs, or concurrent surveys assessing both domestic cats and dogs [1,3,4,6,8–11,13–19,21,25,26,28,30,32,34–37,40,41,43–48]. Apart from infecting both canids and felids, this cestode may also occasionally infect humans [2,24,42].
The intermediate hosts for this parasite are the cat and dog fleas (Ctenocephalides felis and Ctenocephalides canis, respectively), as well as the dog and cat chewing lice, Trichodectes canis, and Felicola subrostratus, respectively [31]. Due to its worldwide distribution, and its ability to infest dogs and cats, the cat flea, C. felis, is considered to be the main intermediate host [5,12,27,46]. Flea larvae ingest D. caninum eggs, with the rate of development in the flea greatly affected by temperature [38,39]. When adults fleas infected with suitably developed metacestodes are ingested by the canine or feline host, the parasite establishes in the small intestine. Here it develops to an adult tapeworm, with shed proglottids visible in faeces from between 17 and 19 days after infection [22,23].
Beugnet et al. [8] investigated the occurrence of D. caninum in fleas from client-owned cats and dogs in Europe, using a new PCR detection assay. The results indicated that easy and regular Dipylidium sp. re-infections of both cats and dogs in European households were likely. Thus, for the first time, the spread of D. caninum between fleas on dogs and cats was confirmed throughout Europe. In this European survey, 2.23% of 1969 cat fleas collected from cats were found to be infected by Dipylidium sp. larvae, compared to 5.2% of 732 cat fleas collected from dogs and 3.1% of 2828 dog fleas collected from dogs. Preliminary analyses performed during this survey, indicated genetic differences between D. caninum metacestodes in fleas collected from dogs and cats, respectively. Low, in 2017, suggested the presence of two clades within D. caninum species [31]. Labuschagne et al., 2018, using the DNA extracted from the initial flea collect from dogs and cats in Europe [8], and adding new fleas collected in the United States, as well as Dipylidium proglottids from Europe, Africa, and Asia, demonstrated the existence of the two distinct genotypes [29]. The initial genetic analysis started in 2012 during an epidemiological survey assessing the infection rate of fleas by Dipylidium caninum using a new PCR probe [8]. In the recent paper, Labuschagne et al., 2018, established a correspondence between the host origin of Dipylidium-infected fleas and the genotype. They demonstrated that the genotypes are not related to geography but to hosts. The so-called feline genotype of D. caninum was found almost exclusively in C. felis collected from cats (95.1%), whereas the so-called canine-genotype was found almost exclusively in C.felis collected from dogs (97.3%), and was the only one observed in C. canis fleas (100%) [29]. The authors also confirmed that the Dipylidium DNA collected by Low et al., (2017) [31], from cat fleas and cat lice collected from cats belong to the feline genotype, and that the Dipylidium caninum collected by East et al., (2013) [17], also belong to the same feline genotype [29,31]. The Dipylidium from the two genotypes are kept in dogs and cats at Clinvet, Bloemfontein, South Africa, allowing the present study.
This paper reports on an in vivo experimental infection study, designed to investigate potential host association with reference to the canine and feline D. caninum genotypes [29].
The objectives of this study were thus two-fold: firstly, to establish whether the two D. caninum genotypes show distinct host interaction, i.e. prepatent period, longevity, and rate of infection; and secondly, to establish whether the genotypes could have sexual reproduction during mixed infection in either dogs or cats.
Materials and Methods
Ethics
The study was approved by the Institutional Animal Care and Use Committee (IACUC). The study conformed to the principles defined and explained in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and its appendix. In addition, the authors have involved the minimum number of animals in the experimental infection study for the purpose of adequate experimental infection model validation. Animals were observed daily for general health, with physical examinations performed by a veterinarian to ensure suitability for inclusion in the study. Throughout the study, the health of the animals was monitored by veterinary personnel. No abnormal clinical signs were observed during either clinical examinations or daily health observations. As a result, none of the animals required concomitant therapy or veterinary care during the study. After termination of the animal phase, the animals received the necessary concomitant therapy (deworming based on praziquantel oral administration), after which they were returned to the Clinvet colony holding facility in order to undergo a resting period.
Study design
The study was designed as a parallel group, non-blinded, randomised, single-centre study, to determine the efficiency in infecting dogs and cats with two different D. caninum genotypes (Figure 1). The D. caninum were sourced from donor cats or dogs, and served to infect fleas. The study was based on an experimental flea infestation model previously published [7,22], in combination with the newly developed PCR hydrolysis probe assay [29].
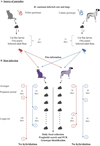 |
Figure 1 Graphical presentation of the experimental study on Dipylidium caninum genotypes and host association. |
Animal details
Three dogs (group 1) and three cats (group 2), all females, were included in the study. The dogs were all beagles, 10 months of age and weighed between 10.20 kg and 11.60 kg. The cats were all domestic shorthair, with ages 7, 9 and 27 months respectively, and body weight ranging between 1.90 kg and 2.92 kg.
Experimental model overview
Cats and dogs were infected concomitantly with both the canine and feline D. caninum genotypes, by means of skin infestation with D. caninum-infected C. felis fleas.
See Fourie et al. [22,23] as well as Beugnet et al. [7] for a detailed description of the experimental infection model employed.
The primary criteria for model validation was a positive result on PCR hydrolysis probe genotyping performed on faeces or positive identification of a D. caninum proglottid collected during macroscopic examination of cages and/or faeces [29]. While both methods constituted confirmation of D. caninum infection, PCR hydrolysis probe genotyping also constituted confirmation of the genotype and hence allowed evaluation of potential genotype host associations. PCR hydrolysis probe genotyping also allowed observation of hybrid DNA patterns. A secondary criterion considered was the duration of proglottid shedding.
Flea infestations and related actions
A first step was to breed two batches of fleas, from eggs to pupae, during approximately 21 days on a flea-rearing medium containing Dipylidium proglottids originating from infected donor cats and dogs with their respective Dipylidium genotype.
A second step before flea infestation of dogs and cats was to perform PCR hydrolysis probe genotyping analyses on the newly emerged fleas to confirm their infection by D. caninum larvae (as well as the D. caninum genotype).
In addition, a sample of 30 fleas from each batch used was dissected and examined microscopically to determine the prevalence of infection with D. caninum metacestodes, as well as their level of development. With reference to the latter, some organisation of the hooklets had to be evident in at least one of the metacestodes present.
Fleas were killed by freezing them, after which they were dissected with the aid of a dissection microscope using two needles. One needle was used to pin the flea down by the thorax, and the other to cut open the tip of the abdomen. Contents were squeezed out using the needle. Metacestodes, if present, were counted and stage of development noted.
The third step consisted in the infestation of dogs and cats with live fleas from the two batches. As fleas containing respective feline and canine genotypes were placed on the animals, it was necessary to have batches with similar Dipylidium sp. infection rates for each infestation.
Thus, after establishing metacestode infection rates, the batches were “diluted” by addition of fleas from a laboratory C. felis flea strain known not to be infected with D. caninum, in order to achieve infection rates that were similar between the two Dipylidium infected flea batches.
Flea infestations were performed on Days 0, 13 and 28. Each cat/dog was skin infested with 200 fleas, including 100 infected fleas (50 with each D. caninum genotype). Animals were allowed to groom freely. Dogs and cats were housed individually during the 168 days of the animal phase.
At Day 56, in order to kill fleas, each animal was treated with an ectoparasiticide (Frontline Plus® for cats and NexGard® for dogs), according to label instructions.
Proglottid collection and analyses
Macroscopic examination of faeces and the individual cages for shed proglottidswas performed at least twice weekly from Days 21 (estimated end of pre-patent period following first flea infestation on Day 0) to Day 168. Collected proglottids were subjected to DNA isolation using a commercial kit. Isolated DNA was subjected to specific PCR amplification of the 28S rDNA region as described by Beugnet et al. [8], followed by genotyping using hydrolysis probes specific for each genotype [29].
PCR hydrolysis probe-based genotyping was used to discriminate between the two identified genotypes exhibiting specific associations towards dogs and cats.
All proglottids were also screened for hybridization using a hybridization probe-based DNA genotyping qPCR assay [29].
Statistical analysis
Seven day periods were used to define “weeks”, as was employed in statistical analyses (Table 1). Weeks were defined by the Investigator based on the pre-patent period of D. caninum, and hence the anticipated commencement of proglottid shedding.
The validity of the experimental model was confirmed based on the positive identification of D. caninum proglottids in faeces.
The success of infection by the D. caninum genotypes was measured by the number of dogs/cats being infected by each genotype, respectively.
For real-time PCR results, canine and feline genotypes were presented descriptively for each three-week interval and overall period. Differences between these genotypes for each interval were compared using a Chi-square test. The level of significance of the formal tests was set at 5% and all tests were two-sided.
The pre-patent period was defined as the number of days from first flea infestation (Day 0) to the first PCR-positive test in proglottids collected from faeces.
The duration of infestation for each D. caninum genotype (worm longevity) was defined as the total number of days where the infestation was regarded as successful, as confirmed by the presence of D. caninum proglottids in faeces and their identification by PCR.
The rate of success, the pre-patent period and the duration of infestation were presented descriptively for cats and dogs, for both the feline and canine D. caninum genotypes respectively, at each assessment time point.
The rate of success was presented using frequencies and percentages, while the duration of the pre-patent period and the duration of infestation were presented using summary statistics (mean, standard deviation, median, minimum and maximum).
SAS Version 9.3 TS Level 1M2 was used for all the statistical analyses.
With reference to sample size, three dogs and three cats were used in this study, which was considered adequate for experimental model method validation using different genotypes. The statistical unit was the individual animal.
Definition of “weeks” (as used in statistical analyses) according to study day periods.
Efficiency of the in vivo experimental model
The model was regarded as effective if host animals challenged with fleas infected with both feline and canine genotypes of D. caninum became infected, as confirmed by expulsion of proglottids and verified by PCR, with either or both genotypes.
Results
Metacestode infection rates
The metacestode infection rates in flea batches placed on animals (obtained through batch dilution with uninfected fleas as described previously), are tabulated in Table 2. Actual metacestode infection rates in the flea batches employed for host infestations ranged between 10% and 33.3%.
Summary of metacestode infection rates of the fleas used, prior to each infestation.
Infection success rates
The rates of D. caninum infection success are presented descriptively for cats and dogs, for both the feline and canine D. caninum genotypes respectively, at each assessment time point, in Table 3.
Rate of Dipylidium caninum infection success (positive animals based on presence of proglottids and positive RLFP results) expressed as frequencies and percentages for the time periods assessed.
Dipylidium sp. infection in dogs
Infections with the canine D. caninum genotype were first observed in all three dogs from Week 5 to 7, with observed infections persisting throughout the study period, while infection with the feline genotype was not observed in all three dogs during that period. Infection with the feline genotype in dogs was observed in 2 out of 3 dogs positive from Week 2 to 4, and then again from Week 17 to 19. However, considering the total period, the three dogs did become infected with the feline strain.
Dipylidium infection in cats
Infections with the feline genotype were first observed in all three cats from Week 2 to 4, with observed infections persisting throughout the study period, while infection with the canine genotype was not observed in all cats during that period. Infections with the canine genotype were first observed in all three cats in group 2 from Week 8 to 10, and then again from Week 17 to 22.
Genotyping results
Hydrolysis probe-based genotyping results are presented in Table 4a (dog group) and Table 4b (cat group). These results confirmed that the canine genotype had a higher frequency of occurrence in dogs, while the feline genotype had a higher frequency of occurrence in cats.
Results (p-values) after statistically comparing the genotyping results for the two groups (Chi-square analysis), with reference to D. caninum genotype employed (either canine or feline), are presented in Table 5.
With the exception of Week 17 to 19, dogs and cats differed significantly with regard to the feline and canine D. caninum genotype frequency of occurrence.
Hydrolysis probe-based genotyping result frequency counts (Dipylidium caninum feline and canine genotypes) in dogs (group 1).
Hydrolysis probe-based genotyping result frequency counts (Dipylidium caninum feline and canine genotypes) for cats (group 2).
Statistical comparison of the cat and dog groups in terms of D. caninum genotypes.
Durations of pre-patent period
Durations of the pre-patent period are presented in Table 6a (dog group) and Table 6b (cat group), using summary statistics (mean, standard deviation, median, minimum and maximum). In dogs, the average pre-patent period was shorter for the canine genotype (i.e. 38 days) compared to the feline genotype (70 days), while the opposite was true in cats (34 days for feline genotype versus 53 days for canine genotype). With 3 animals in each group, these differences were not significant.
Individual and summary statistics of pre-patent periods (in days) for dogs (group 1).
Individual and summary statistics of pre-patent periods (in days) for cats (group 2).
Durations of infestation
Durations of infestation are presented descriptively for cats and dogs, for both the feline and canine D. caninum genotypes, respectively in Table 7a (group 1) and Table 7b (group 2). In dogs, the observed infection with the canine genotype persisted longer compared to the feline genotype (91 days versus 24 days), while the opposite was true for cats (130 days for the feline genotype compared to 41 days for the canine one). These differences were significant.
Individual and summary statistics of duration of Dipylidium infection (in days) for dogs (group 1).
Individual and summary statistics of duration of Dipylidium sp. infection (in days) for cats (group 2).
Hybridization
No sign of hybridization between D. caninum genotypes was detected for any of the proglottid specimen samples analyzed. This demonstrates that no hybrid proglottid-containing eggs were observed, despite the six mixed infections (three in dogs, three in cats) allowing potential sexual reproduction between adult Dipylidium sp. in the intestine.
Discussion
The experimental infection model based on infected flea challenges has previously been used with great success in several efficacy studies [7,22,23]. The molecular characterization of D. caninum isolates collected from dogs, cats, and in infected fleas collected either from dogs or cats enabled the identification of two distinct genotypes that clearly differ from each other [29]. Previous studies had also suggested the existence of different genetic profiles, or suggested that there could be clades or even different species under the name Dipylidium caninum [31].
East et al., 2013, collected Dipylidium caninum proglottids from six spotted hyena [17]. They used one of these samples to obtain 12S rRNA fragments (314 bp and 1176 bp). When comparing their 314 bp sequence data with two published D. caninum sequences of the same fragment, they obtained a high (99%) similarity to one sequence from Europe (accession number L49460.1) but a considerably lower similarity (89%) to one sequence from Asia (accession number AB031362.1). When they compared the available 1176 bp sequence (accession number KF202097) to their similar fragment from D. caninum, they obtained a relatively low similarity (89%). By looking at their sequences and comparison to the complete mitochondrial (mt) sequences of the D. caninum feline genotype (MG587892), Labuschagne et al., obtained 99.1% identity between the D. caninum isolated from the hyena (KF202097) and the D. caninum feline genotype (MG567892) isolated from a cat [29]. When comparing to the mt genome of the Dipylidium dog genotype, there was only around 88.5% identity [29]. More recently, Low et al., (2017) [31], collected ectoparasites from dogs and cats in Malaysia. In this study, Ctenocephalides felis (92 specimens) and Felicola subrostratus (30 specimens) were collected from 20 cats. PCR amplification utilizing the primers published in 2014 [8] was performed for the 28S rRNA gene region of Dipylidium. Low et al. also characterized the positive samples with a 12S rRNA gene amplification [31]. They found that the representative 28S rRNA sequence isolated from their flea and louse specimens (GenBank accession no. KY751956) demonstrated 95% sequence similarity with that of D. caninum (GenBank accession no. AF023120), and they suggested the existence of two distinct clades within Dipylidium caninum. They concluded that their 12S rRNA sequences (GenBank accession no. KY751955) were identical to the spotted hyena isolate from East et al. (GenBank accession no. KF202097) [31]. Labuschagne et al. compared the 12S mt rDNA sequence of the feline and canine genotypes to the D. caninum 12S mt rDNA sequences used by Low et al. [29]. The Dipylidium DNA isolates collected from cat fleas and cat louse from cats in Malaysia were identical to the D. caninum feline genotype [29]. The hypothesis drawn by Low et al. [31] on the existence of two clades is thus confirmed by the work of Labuschagne et al., the proposed clades corresponding to the canine and feline Dipylidium genotypes [29].
These two genotypes are not related to geographical origin, as they were found on all continents (i.e. North America, Europe, Asia, and Africa), but clearly to their host origin, dogs or cats (and hyena). Nevertheless, the specificity is not absolute, as we were able to infect cats and dogs with both genotypes during the present experimental study. Labuschagne et al., studying the fleas collected in 2012 [8], indicated that around 10% of the cat fleas collected on cats and 2% of the cat fleas collected on dogs, were infected with the other genotype than the host-genotype. The common presence of both cats and dogs in the same households, being infested by the same flea species (i.e. Ctenocephalides felis), could explain the infection of cats and dogs by both genotypes, but the different observed prevalences suggested biological adaptation, hence the decision to conduct the present study. On the other hand, C. canis and P. irritans fleas being more specific to dogs, 100% of the infected fleas were found to harbour the canine genotype of Dipylidium caninum [29].
The results obtained during the experimental infections demonstrated significant biological variations between the two genotypes in regard to their host association. The pre-patent periods were significantly shorter for the canine genotype in dogs and the feline genotype in cats, respectively. The duration of proglottid shedding (i.e. patent period or longevity) was significantly longer for the canine genotype in dogs and the feline genotype in cats, thus confirming biological variations and the host specificity for each genotype. The canine D. caninum genotype occurred at a significantly higher frequency in dogs, and the feline genotype at a significantly higher frequency in cats. Nevertheless, the host tropism was not absolute as both canine and feline genotypes were diagnosed in cats and dogs, respectively.
Even though Cyclophyllidea cestodes are hermaphrodites and present auto-fertilization, cross-fertilization is described in the presence of several adults at the same place [20,33]. Under our experimental conditions, despite mixed infections, no hybrid DNA was observed in single proglottids, demonstrating the absence of hybrid eggs.
Genomic and mitochondrial sequencing, combined with an in vivo experimental study and novel PCR hydrolysis probe genotyping assay, demonstrated that the two distinct D. caninum genotypes [29] present significant biological differences with a specific host association. A species is classically defined by individuals being able to reproduce together. The absence of hybrid eggs raises the question of the species level of each Dipylidium caninum genotype. Dipylidium caninum Linnaeus 1758 has originally been described in dogs. Another study is planned to assess the possible presence of morphological differences in addition to the genetic and biological observations. The current results, on both the genetic and the biological aspects, raise the question of the possible existence of two host-associated species inside the genus Dipylidium.
Conflicts of interest
The authors declare that they have no conflicts of interest in relation to this article.
Acknowledgements
The authors would like to thank the research personnel from both Clinomics and Clinvet International (Pty) Ltd for their assistance in the conduct of the research. The study was funded by Merial SAS, France.
References
- Abdybekova AM, Torgerson PR. 2012. Frequency distributions of helminths of wolves in Kazakhstan. Veterinary Parasitology, 184, 348-351. [CrossRef] [PubMed] [Google Scholar]
- Adam AA, Saeed OM, Ibrahim HM, El HY, Ahmed ME. 2012. Case report. Dipylidium caninum infection in a 41-year-old Sudanese man in Nyala, Sudan: The first reported case in Sudan in 2006. Al Neelain Medical Journal, 2(6), 37-42. [Google Scholar]
- Al-Sabi MNS, Chriél M, Jensen TH, Enemark HL. 2013. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009-2012 - A comparative study. International Journal for Parasitology: Parasites and Wildlife, 2, 144-151. [CrossRef] [Google Scholar]
- Anene BM, Nnaji TO, Chime AB. 1996. Intestinal parasitic infections of dogs in the Nsukka area of Enugu State, Nigeria. Preventive Veterinary Medicine, 27, 89-94. [CrossRef] [Google Scholar]
- Beck W, Boch K, Mackensen H, Wiegand B, Pfister K. 2006. Qualitative and quantitative observations on the flea population dynamics of dogs and cats in several areas of Germany. Veterinary Parasitology, 137, 130-136. [CrossRef] [PubMed] [Google Scholar]
- Beiromvand M, Akhlaghi L, Fattahi Massom SH, Meamar AR, Motevalian A, Oormazdi H, Razmjou E. 2013. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Preventive Veterinary Medicine, 109, 162-167. [CrossRef] [Google Scholar]
- Beugnet F, Delport P, Luus H., Crafford D, Fourie J. 2013. Preventive efficacy of Frontline® Combo and Certifect® against Dipylidium caninum infestation of cats and dogs using a natural flea (Ctenocephalides felis). Parasite, 20, 7. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Beugnet F, Labuschagne M, Fourie J, Jacques G, Farkas R, Cozma V., Halos L, Hellmann K, Knaus M, Rehbein S. 2014. Occurrence of Dipylidium caninum in fleas from client-owned cats and dogs in Europe using a new PCR detection assay. Veterinary Parasitology, 205, 300-306. [CrossRef] [PubMed] [Google Scholar]
- Bwalya EC, Nalubamba KS, Hankanga C, Namangala B. 2011. Prevalence of canine gastrointestinal helminths in urban Lusaka and rural Katete Districts of Zambia. Preventive Veterinary Medicine, 100, 252-255. [CrossRef] [PubMed] [Google Scholar]
- Cabrera PA, Parietti S, Haran G, Benavidez U, Lloyd S, Perera G, Valledor S, Gemmell MA, Botto T. 1996. Rates of reinfection with Echinococcus granulosus, Taenia hydatigena, Taenia ovis and other cestodes in a rural dog population in Uruguay. International Journal for Parasitology, 26, 79-83. [CrossRef] [PubMed] [Google Scholar]
- Calvete C, Lucientes J, Castillo JA, Estrada R, Gracia MJ, Peribáñez MA, Ferrer M. 1998. Gastrointestinal helminth parasites in stray cats from the mid-Ebro Valley, Spain. Veterinary Parasitology, 75, 235-240. [CrossRef] [PubMed] [Google Scholar]
- Chandra S, Forsyth M, Lawrence AL, Emery D, Šlapeta J. 2017. Cat fleas (Ctenocephalides felis) from cats and dogs in New Zealand: Molecular characterisation, presence of Rickettsia felis and Bartonella clarridgeiae and comparison with Australia. Veterinary Parasitology, 234, 25-30. [CrossRef] [PubMed] [Google Scholar]
- Dai RS, Li ZY, Li F, Liu DX, Liu W, Liu GH, He SW, Tan MY, Lin RQ, Liu Y, Zhu XQ. 2009. Severe infection of adult dogs with helminths in Hunan Province, China poses significant public health concerns. Veterinary Parasitology, 160, 348-350. [CrossRef] [PubMed] [Google Scholar]
- Dalimi A, Sattari A, Motamedi G. 2006. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Veterinary Parasitology, 142, 129-133. [CrossRef] [PubMed] [Google Scholar]
- Dubná S, Langrová I, Nápravník J, Jankovská I, Vadlejch J, Pekár S, Fechtner J. 2007. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Veterinary Parasitology 145, 120-128. [CrossRef] [PubMed] [Google Scholar]
- Dybing NA, Fleming PA, Adams PJ. 2013. Environmental conditions predict helminth prevalence in red foxes in Western Australia. International Journal for Parasitology: Parasites and Wildlife, 2, 165-172. [CrossRef] [Google Scholar]
- East ML, Kurze C, Wilhelm K, Benhaiem S, Hofer H. 2013. Factors influencing Dipylidium sp. infection in a free-ranging social carnivore, the spotted hyaena (Crocuta crocuta). International Journal for Parasitology: Parasites and Wildlife, 2, 257-265. [CrossRef] [Google Scholar]
- Eguía-Aguilar P, Cruz-Reyes A, Martínez-Maya JJ. 2005. Ecological analysis and description of the intestinal helminths present in dogs in Mexico City. Veterinary Parasitology, 127, 139-146. [CrossRef] [PubMed] [Google Scholar]
- El-Seify MA, Aggour MG, Sultan K, Marey NM. 2017. Gastrointestinal helminths of stray cats in Alexandria, Egypt: A fecal examination survey study. Veterinary Parasitology: Regional Studies and Reports, 8, 104-106. [CrossRef] [Google Scholar]
- Euzéby J. 1966. Cycles évolutifs, Copulation et Fécondation, in Les maladies vermineuses des animaux domestiques et leur incidence sur la pathologie humaine. Tome II Maladies dues aux plathelminthes. Fascicule premier Cestodes, Vigot Frères, Editor. Vigot Frères : Paris, France. p. 39-40. [Google Scholar]
- Fontanarrosa MF, Vezzani D, Basabe J, Eiras DF. 2006. An epidemiological study of gastrointestinal parasites of dogs from Southern Greater Buenos Aires (Argentina): Age, gender, breed, mixed infections, and seasonal and spatial patterns. Veterinary Parasitology 136, 283-295. [CrossRef] [PubMed] [Google Scholar]
- Fourie JJ, Crafford D, Horak IG, Stanneck D. 2012. Prophylactic treatment of flea-infested cats with an imidacloprid/flumethrin collar to forestall infection with Dipylidium caninum. Parasites & Vectors, 5, 151. [CrossRef] [PubMed] [Google Scholar]
- Fourie JJ, Crafford D, Horak IG, Stanneck D. 2013. Prophylactic treatment of flea-infested dogs with an imidacloprid / flumethrin collar (Seresto, Bayer) to preempt infection with Dipylidium caninum. Parasitology Research, 112, 33-46. [CrossRef] [PubMed] [Google Scholar]
- García-Agudo L, García-Martos P, Rodríguez-Iglesias M. 2014. Dipylidium caninum infection in an infant: a rare case report and literature review. Chinese Journal of Schistosomiasis Control, 26, 565-567. [Google Scholar]
- Gates MC, Nolan TJ. 2009. Endoparasite prevalence and recurrence across different age groups of dogs and cats. Veterinary Parasitology, 166, 153-158. [CrossRef] [PubMed] [Google Scholar]
- Johnson SAM, Gakuya DW, Mbuthia PG, Mande JD, Maingi N. 2015. Prevalence of gastrointestinal helminths and management practices for dogs in the Greater Accra region of Ghana. Heliyon, 1(1), e00023. [CrossRef] [Google Scholar]
- Kamani J, Baneth G, Gutiérrez R, Nachum-Biala Y, Salant H, Mumcuoglu KY, Harrus S. 2015. Molecular screening of Ctenocephalides felis fleas collected from stray cats in the Jerusalem District, Israel, for Bartonella spp., Rickettsia spp. and Coxiella burnetii. Veterinary Parasitology: Regional Studies and Reports, 1, 59-64. [CrossRef] [Google Scholar]
- Labarthe N, Serrão ML, Ferreira AMR, Almeida NKO, Guerrero J. 2004. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Veterinary Parasitology, 123, 133-139. [CrossRef] [PubMed] [Google Scholar]
- Labuschagne M, Beugnet F, Rehbein S, Guillot J, Fourie J, Crafford D. 2018. Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas. Part 1. Molecular characterization of Dipylidium caninum: genetic analysis supporting two distinct species adapted to dogs and cats. Parasite, 25, 30. [Google Scholar]
- Lahmar S, Boufana B, Ben Boubaker S, Landolsi F. 2014. Intestinal helminths of golden jackals and red foxes from Tunisia. Veterinary Parasitology, 204, 297-303. [CrossRef] [Google Scholar]
- Low VL, Prakash BK, Tan TK, Sofian-Azirun M, Anwar FHK, Vinnie-Siow WY, AbuBakar S. 2017. Pathogens in ectoparasites from free-ranging animals: Infection with Rickettsia asembonensis in ticks, and a potentially new species of Dipylidium in fleas and lice. Veterinary Parasitology, 245, 102-105. [CrossRef] [PubMed] [Google Scholar]
- Martínez-Moreno FJ, Hernández S, López-Cobos E, Becerra C, Acosta I, Martínez-Moreno A. 2007. Estimation of canine intestinal parasites in Córdoba (Spain) and their risk to public health. Veterinary Parasitology, 143, 7-13. [CrossRef] [PubMed] [Google Scholar]
- Mehlhorn H. 2000. Eucestoda, in Encyclopedic reference of Parasitology: Biology, Structure, Function, 3rd Edn, Mehlhorn H, Editor. Springer: Berlin, Germany. p. 223-222. [Google Scholar]
- Mohd Zain SN, Sahimin N, Pal P, Lewis JW. 2013. Macroparasite communities in stray cat populations from urban cities in Peninsular Malaysia. Veterinary Parasitology, 196, 469-477. [CrossRef] [PubMed] [Google Scholar]
- Moskvina TV, Zheleznova LV. 2015. A survey on endoparasites and ectoparasites in domestic dogs and cats in Vladivostok, Russia 2014. Veterinary Parasitology: Regional Studies and Reports, 1-2, 31-34. [CrossRef] [Google Scholar]
- Oliveira-Sequeira T, Amarante A, Ferrari T, Nunes L. 2002. Prevalence of parasites in dogs from Sao Paulo state, Brazil. Veterinary Parasitology, 103, 19-27. [CrossRef] [Google Scholar]
- Papazahariadou M, Founta A, Papadopoulos E, Chliounakis S, Antoniadou-Sotiriadou K, Theodorides Y. 2007. Gastrointestinal parasites of shepherd and hunting dogs in the Serres Prefecture, Northern Greece. Veterinary Parasitology, 148, 170-173. [CrossRef] [PubMed] [Google Scholar]
- Pugh RE, Moorhouse DE. 1985. Factors affecting the development of Dipylidium caninum in Ctenocephalides felis felis (Bouché, 1835). Zeitschrift für Parasitenkunde, 71, 765-775. [CrossRef] [Google Scholar]
- Pugh RE. 1987. Effects on the development of Dipylidium caninum and on host reaction to this parasite in the adult flea (Ctenocephalides felis felis). Parasitology Research, 73, 171-177. [CrossRef] [PubMed] [Google Scholar]
- Richards DT, Harris S, Lewis, JW. 1995. Epidemiological studies on intestinal helminth parasites of rural and urban red foxes (Vulpes vulpes) in the United Kingdom. Veterinary Parasitology, 59, 39-51. [CrossRef] [PubMed] [Google Scholar]
- Riggio F, Mannella R, Ariti G, Perrucci S. 2013. Intestinal and lung parasites in owned dogs and cats from central Italy. Veterinary Parasitology, 193, 78-84. [CrossRef] [PubMed] [Google Scholar]
- Sahin I, Köz S, Atambay M, Kayabas U, Piskin T, Unal B. 2015. A rare cause of diarrhea in a kidney transplant recipient: Dipylidium caninum. Transplantation Proceedings, 47, 2243-2244. [CrossRef] [PubMed] [Google Scholar]
- Shin JW, Liao WT. 2002. Humoral immune response to Dipylidium caninum infection of stray dogs in Taiwan. Veterinary Parasitology, 104, 351-356. [CrossRef] [PubMed] [Google Scholar]
- Soran MM, Ciopasiu RM, Ionita M, Mitrea IL. 2015. Investigation of endoparasite community of dogs and cats in urban areas in South-Eastern Romania: Potential risks for animal and public health. Journal of Biotechnology 208, S96. [CrossRef] [Google Scholar]
- Soriano SV, Pierangeli NB, Roccia I, Bergagna HFJ, Lazzarini LE, Celescinco A, Saiz MS, Kossman A, Contreras PA, Arias C, Basualdo JA. 2010. A wide diversity of zoonotic intestinal parasites infects urban and rural dogs in Neuquén, Patagonia, Argentina. Veterinary Parasitology, 167, 81-85. [CrossRef] [PubMed] [Google Scholar]
- Takeuchi-Storm N, Mejer H, Al-Sabi MNS, Olsen CS, Thamsborg SM, Enemark HL. 2015. Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration McMaster technique. Veterinary Parasitology, 214 (3-4), 327-332. [CrossRef] [PubMed] [Google Scholar]
- Traub RJ, Robertson ID, Irwin PJ, Mencke N, Thompson RCA. 2005. Canine gastrointestinal parasitic zoonoses in India. Trends in Parasitology, 21, 42-48. [CrossRef] [PubMed] [Google Scholar]
- Ziadinov I, Deplazes P, Mathis A, Mutunova B, Abdykerimov K, Nurgaziev R, Torgerson PR. 2010. Frequency distribution of Echinococcus multilocularis and other helminths of foxes in Kyrgyzstan. Veterinary Parasitology, 171, 286-292. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Beugnet F, Labuschagne M, Vos Cd, Crafford D, Fourie J. 2018. Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas. Parasite 25, 31
All Tables
Definition of “weeks” (as used in statistical analyses) according to study day periods.
Summary of metacestode infection rates of the fleas used, prior to each infestation.
Rate of Dipylidium caninum infection success (positive animals based on presence of proglottids and positive RLFP results) expressed as frequencies and percentages for the time periods assessed.
Hydrolysis probe-based genotyping result frequency counts (Dipylidium caninum feline and canine genotypes) in dogs (group 1).
Hydrolysis probe-based genotyping result frequency counts (Dipylidium caninum feline and canine genotypes) for cats (group 2).
Statistical comparison of the cat and dog groups in terms of D. caninum genotypes.
Individual and summary statistics of pre-patent periods (in days) for dogs (group 1).
Individual and summary statistics of pre-patent periods (in days) for cats (group 2).
Individual and summary statistics of duration of Dipylidium infection (in days) for dogs (group 1).
Individual and summary statistics of duration of Dipylidium sp. infection (in days) for cats (group 2).
All Figures
 |
Figure 1 Graphical presentation of the experimental study on Dipylidium caninum genotypes and host association. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


