| Issue |
Parasite
Volume 20, 2013
|
|
|---|---|---|
| Article Number | 35 | |
| Number of page(s) | 12 | |
| DOI | https://doi.org/10.1051/parasite/2013033 | |
| Published online | 15 October 2013 | |
urn:lsid:zoobank.org:pub:E52C2F06-AE58-4074-99A3-497AB27C1EE2
Research Article
A new flea, Ectinorus (Ectinorus) insignis n. sp. (Siphonaptera, Rhopalopsyllidae, Parapsyllinae), with notes on the subgenus Ectinorus in Chile and comments on unciform sclerotization in the superfamily Malacopsylloidea
Description d’une puce nouvelle, Ectinorus (Ectinorus) insignis n.sp. (Siphonaptera, Rhopalopsyllidae, Parapsyllinae): notes sur le sous-genre Ectinorus au Chili et discussion sur les sclérotisations unciformes dans la super-famille Malacopsylloidea
1
Laboratoire de Parasitologie et Zoologie appliquée, Faculté de Médecine, 2 avenue du Professeur Léon Bernard, 35043 Rennes Cedex, France
2
Institut de Parasitologie de l’Ouest, Faculté de Médecine, 2 avenue du Professeur Léon Bernard, 35043 Rennes Cedex, France
3
Laboratoire de Parasitologie, Mycologie et Immunologie parasitaire, Centre Hospitalier Régional Universitaire, 2 rue Henri Le Guilloux, 32033 Rennes Cedex, France
4
Facultad de Ciencias Veterinarias, Universitad de Concepción, Casilla 537, Chillán, Chile
* Corresponding author: jc.beaucournu@gmail.com
Received:
6
March
2013
Accepted:
10
September
2013
A list is provided for the species of Ectinorus sensu stricto from Chile. Ectinorus (Ectinorus) insignis n. sp. is described from Chile: this species is characterized by the male genitalia. In the subgenus Ectinorus, the authors report the presence in Chile of E. pilosus Beaucournu & Carmen Castro, 2002 described from Argentina and E. simonsi (Rothschild, 1904) described from Bolivia but also known from Peru. A female neallotype is designated for E. ineptus Johnson, 1957. “Unciform sclerotization” is noted and illustrated for the first time, in all Malacopsylloidea, and a list is given for all studied species.
Résumé
La liste des Ectinorus sensu stricto connus du Chili est donnée et Ectinorus (Ectinorus) insignis n. sp. est décrit: l’espèce est caractérisée par la structure des genitalia mâles. Dans le sous-genre Ectinorus, les auteurs signalent la présence au Chili de E. pilosus Beaucournu & Carmen Castro, 2002, décrit d’Argentine et de E. simonsi (Rothschild, 1904) décrit de Bolivie, puis retrouvé au Pérou. Un néallotype femelle est désigné pour E. ineptus Johnson, 1957. Une “sclérification unciforme” est notée et illustrée pour la première fois chez tous les Malacopsylloidea, et une liste est donnée pour toutes les espèces étudiées.
Key words: Siphonaptera / Malacopsylloidea / Rhopalopsyllidae / Chile / New species
ZooBank Author ID:
Jean-Claude Beaucournu – urn:lsid:zoobank.org:author:BA6F408D-0C0A-41E5-9DFB-D792B028EF15
Sorya Belaz – urn:lsid:zoobank.org:author:4E5C5295-A4FB-493D-A848-EA6332674633
Sebastián Muñoz-Léal – urn:lsid:zoobank.org:author:565FC990-8EE3-40F0-8181-8B50DACBF5F0
Daniel González-Acuña – urn:lsid:zoobank.org:author:6AA03FF4-6488-4480-8E43-2FEFB9B85177
© J.-C. Beaucournu et al., published by EDP Sciences, 2013
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The interest of the genus Ectinorus (and the related genera Delostichus, Tetrapsyllus, etc.), regardless of its morphological features, is that it is one of the bubonic plague vectors, transmitting Yersinia pestis in Chile-Andean subregion (e.g., Macchiavello, 1948) [28]. Admittedly, the importance of plague in the southern cone of South America is of lesser importance than in the Old World (Kazakhstan, Mongolia, China, Vietnam, Republic of Congo, Tanzania, Madagascar), but the potential for disease remains and a knowledge of endemic fleas is important.
Genus Ectinorus Jordan, 1942
The genus Ectinorus (Rhopalopsyllidae: Parapsyllinae), endemic to the Chile-Andean subregion, includes related sigmodontine rodents fleas. Three subgenera are included in Smit’s review (1987) [36]: Panallius Jordan, 1942, Ectinorus sensu stricto and Ichyonus Smit, 1987. Until now 33 Ectinorus sensu stricto species were known (Hastriter & Sage, 2009) [16]. Fifteen species occur in Chile (those for which type localities are in Chile are marked with an asterisk) and include (listed alphabetically): E. chilensis* Lewis, 1976, E. cocyti* (Rothschild, 1904), E. curvatus* Beaucournu & Gallardo, 1991, E. gallardoi* Hastriter, 2001, E. ineptus Johnson, 1957 (in Beaucournu & Gallardo, 1991) [5], E. ixanus (Jordan, 1942) (in Beaucournu & Kelt, 1990) [9], E. lagidium* Beaucournu & Gonzàlez, 2005, E. levipes (Jordan & Rothschild, 1923) (in Beaucournu & Kelt, 1990), E. martini* Lewis, 1976, E. mimacydis* Beaucournu & Gallardo, 2004, E. mondacai* Hastriter, 2001, E. nomisis Smit, 1987 (in Beaucournu & Gallardo, 1989: description of female neallotype) [4], E. setosicornis Jordan, 1942 (in Beaucournu & Gallardo, 1991) [5], E. splendidus* Smit, 1968 and E. uncinatus* Beaucournu & Gallardo, 1991.
An additional three species are here reported from Chile for the first time: Ectinorus pilosus Beaucournu & Carmen Castro, 2002 [2] described from Argentina, E. simonsi (Rothschild, 1904) [32] known previously from Bolivia and Peru, and a new species, Ectinorus insignis Beaucournu & Gonzàlez-Acuña. In addition, a neallotype is designated for the female of Ectinorus ineptus Johnson, 1957 [19]. The total number of Ectinorus sensu stricto known from Chile is elevated to 18 species.
Ectinorus (Ectinorus) pilosus Beaucournu & Carmen Castro, 2002
-
Ectinorus pilosus Beaucournu & Carmen Castro, 2002: Description of holotype male and allotype female, Santa Maria (26° 40′ S–66° 02′ W) (Catamarca), Argentina, on Ctenomys sp. (later determined as Ctenomys knighti Thomas, 1919), collection date unknown.
For this species, one male and one female were found in Chile, Copiapó (Atacama), on Ctenomys sp., collection data unknown.
Ectinorus (Ectinorus) simonsi (Rothschild, 1904)
-
Pulex simonsi (Rothschild, 1904): two males and one female on Neoctodon simonsi (= Octodontomys gliroides) at Challapata (Avaroa, Bolivia), X/11/1901.
-
Rhopalopsyllus simonsi (Rothschild): Jordan & Rothschild, 1908 [23]: redescription.
-
Dysmicus acheronis Johnson, 1957 [19]: holotype male, Yuru (Arequipa, Peru) on Galea musteloides, VIII/8/1939. Smit (1987): acheronis synonymized with simonsi.
-
Ectinorus (Ectinorus) simonsi (Rothschild, 1904): drawings of male and female on Lagidium viscacia and a new locality is reported: Zudañez, Bolivia (in the Bolivian subregion, province of Jaime Zudañez), IV/19/1955 (Smit, 1987) [36].
One male found in Chile, Chusmiza (19° 40′ S–69° 10′ W), altitude 3430 m (Tarapaca), on Octodontomys gliroides, X/15/2011 (D. Gonzàlez-Acuña rec.). Identification is obvious for this species (apex of aedeagus, sternum VIII) but in this area on October 9, 1989, we found on the same host, at 2500 m altitude, three male intergrades between simonsi and nomisis Smit, 1987. These taxa are so close that it is likely that E. nomisis is a subspecies of E. simonsi.
Ectinorus (Ectinorus) ineptus Johnson, 1957
-
Ectinorus ineptus Johnson, 1957: description of male holotype (plates 67–69) at Picotani (Puno, Peru), on Phyllotis (Auliscomys) pictus, IX/14/1941.
-
Ectinorus (Ectinorus) ineptus Johnson 1957: Smit (1987) illustrated the genitalia of the male holotype (their Figures 189 and 190), from Auliscomys pictus.
-
Ectinorus (Ectinorus) ineptus Johnson, 1957, Beaucournu & Gallardo, 1991 [5]: Parinacota (18° 12′ S–69° 16′ W), altitude 4000 m, Chile, on Phyllotis darwini, IX/30/1989. One male and one female: Short description of the male and the female (Figures 6, 9, 12–14), female explicitly “not designated as neallotype because two other very close taxa have been collected in the same region syntopically with this species (e.g., E. curvatus n. sp.)”.
-
Ectinorus (Ectinorus) ineptus Johnson, 1957: Lake Chungara (18° 15′ S–69° 10′ W), altitude 4574 m, Chile (Parinacota) on Auliscomys boliviensis (Waterhouse, 1846), one male and one female XI/21/2011; Enquelga, altitude 3700 m, Chile (Tarapaca) on Eligmodontia puerulus (Philipi, 1896), one female X/17/2011 (D. Gonzàlez-Acuña rec.) (The determinations of all Chilean hosts are after Wilson & Reeder, 2005) [37].
So we have two pairs, each in syntopy on the same individual host and a sole female: therefore, the identity of the female drawn in 1991 is confirmed. Currently males, in Chile, have been collected from two locations close to each other.
We select the 1991 female, as neallotype, because the others have misplaced spermatheca, but are otherwise identical. So the neallotype is from Parinacota (18° 12′ S–69° 16′ W), altitude 4000 m, Chile (Parinacota) on Phyllotis darwini, IX/30/1989.
Description of females
Material examined: two males and three females, as indicated above.
Material deposited: Neallotype in the collection of first author (Collection JCB currently in Faculty of Medicine, Rennes), for ultimate deposition in Muséum National d’Histoire Naturelle, Paris, France (MNHN).
Head capsule: With well-developed tubercle, without micro-seta visible in the vallum. Genal margin slightly convex; maxillary palpus with segments I, II and IV of same length; segment III shorter; labial palpus with seven segments, not reaching apex of coxa; pre-antennal seta very small; six marginal setae and two small, thicker setae in posterior point of the gena; two occipital rows, respectively, of one and five setae.
Thorax: Prothorax with anterior row of five small setae and posterior row of seven with intercalaries. Mesothorax: 14 small setae and principal row of 6 longer setae; 10 pseudosetae. Metathorax: four anterior setae and posterior row of seven longer setae, most dorsal seta curved. Metepimeron with four long setae; spiracle pointed, or shaped as a “nightcap”. Femoral guard setae long; external half the length of internal. Tibia with eight notches on dorsal margin. Notches II, V and VIII with, at least, one long seta. Longest seta of notch VIII extends to apex of first tarsomere. Longest seta of this tarsomere extends to apex of tarsomere II; longest seta of tarsomere II extends to half of the length of distitarsomere.
Abdominal segments: Tergum I with eight spinelets, four on each side. Tergum II with row of seven long setae, most ventral seta at level of spiracle. Same setation on terga III to VII, but most ventral seta below spiracles; spiracles relatively large and rounded. Tergum VIII (Figure 2) with small area of seven or eight small setae anterior to spiracle; spiracle marginal and vermiform. Unciform sclerotization present (see below). Anal stylet (Figure 1) long, conical, with one small seta below insertion of long apical seta. Below ventral anal segment four long marginal setae and 16–18 lateral setae of various lengths and seven to eight thin, small mesal setae. Sternum II with 1 barely discernible striarium and laterally 17 thin lateral setae and 2 small marginal setae. Sternum III with lateral row of six setae and three to four dispersed setae. Sterna IV and V with main row of four or five setae and one isolated seta. Sternum VI with simple row of five setae. Sternum VII (Figure 2) with row of six setae inserted on half of the segment. Margin gently convex. Sternum VIII (Figure 2) large with four to five apical micro-setae.
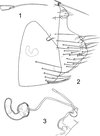 |
Figures 1–3. Ectinorus (Ectinorus) ineptus (Johnson, 1957) neallotype. 1: anal stylet; 2: terminal segment and unciform sclerotization; 3: spermatheca and ducti. |
Spermatheca: Bulga angular (Figure 3), slightly wider than long; hilla slightly curved, twice as long as bulga; ductus obturatus and ductus spermathecae of same width, the d. s. twice as long as the d. o. Typically, for this group, ductus bursa appears as a curved “ε”.
Dimensions (slide-mounted insects in personal collection): males 2.1–2.8 mm (holotype 2.7), females 2.5–2.8 mm.
Specificity: Auliscomys Osgood, 1915 (Rodentia – Cricetidae – Sigmodontinae) must be the elective type host-genus; it is taxonomically very close to Phyllotis Waterhouse, 1837.
Ectinorus (Ectinorus) insignis Beaucournu & González-Acuña n. sp.
urn:lsid:zoobank.org:act:31EF7F63-C723-4961-8628-7548325BEAB6
Authorship: Note that the authors of the new taxon are different from the authors of this paper; Article 50.1 and Recommendation 50A of International Code of Zoological Nomenclature.
Material examined: Holotype male on Eligmodontia puerulus (Philippi, 1896) (Rodentia – Cricetidae – Sigmodontinae), Toconao (23° 07′ S–68° 00′ O), altitude 2469 m, (El Loa), Chile, X/11/2011. Allotype female in syntopy with the holotype (D. González-Acuña rec.).
Material deposited: Types in the collection of first author (Collection JCB, currently in Faculty of Medicine, Rennes), for ultimate deposition in Muséum National d’Histoire Naturelle, Paris (MNHN).
Etymology: from the Latin word insignis derived from in signum “showing a particular character”, this in reference to the male genitalia.
Description
Ectinorus insignis n. sp. has a well-developed processus basimeris ventralis and belongs to the “hapalus-group” (Smit, 1987) [36]. From a dichotomous view, this character is not chosen by this author who uses the presence or absence of spinelets on tergum I.
Head capsule: frontal tubercle not very prominently situated a short distance from the labial angle. Maxillary and labial palps extend slightly beyond the apex of trochanter. Segments I and III of maxillary palpus are the same length but slightly shorter than II or IV. Labial palpus with eight segments, including basal segment. Pre-antennal seta of male well developed, but very thin and small in female. Three pre-ocular setae, the ventral one longer as typical. Eye pigmented, indented and with sclerotized “bridge” near the gena. One post-ocular seta, three marginal setae and one very small spiniform seta on angle between gena and antennal fossa. three occipital rows with one, one and four setae in the male but only two rows in the female of one and four setae.
Thorax: prothorax with one marginal row of six well-developed setae and one anterior row of three. Male with single pseudoseta, female with two. Mesothorax with one marginal row of four long setae and anteriorly three to four small ones; one row of pseudosetae, 13 in the male, 12 in the female. Metathorax with one marginal row of four well-developed setae. Metepimeron with four setae and the spiracle shaped like a “nightcap”. Femur I: femoral guard setae very unequal in length (internal seta length and thickness twice that of the external one). Femur III: internal femoral-tibia guard seta four times as long as the external one but only slightly thicker. Tibia III with six dorsal notches bearing setae, the second, fourth and sixth with at least one long slender seta. First tarsomere III with a long apical seta as long as this segment. Second tarsomere III with a tuft of three long setae, the longest extending beyond the apex of the distitarsomere in the male and half that length in the female.
Abdomen (unmodified segments): male tergum I without spinelet while the female has one or two small spinelets. Terga II–VII, one principal row of six setae, the most ventral at level of spiracle in the male but below the spiracle in the female. Spiracles practically round in the male whereas shaped like a «nightcap» in the female. One ante-sensilial seta; marginal in the female, as typical. Sensilium typical with 17–18 pits. Sternum II with two thin marginal setae; three thin lateral setae (male) or 16–18 (female). Striarium absent. Sterna III and IV with row of three thin setae (male) or two to three (female). Sterna V–VII with one row of two thin setae (male) or respectively three, five and six long, thin and gently curved setae (female).
Abdomen (male genital segments): Sternum VIII (Figure 4) with inferior-distal lobe as in numerous species of Ectinorus. Lobe, circular with two small curved setae on its posterior margin, linked to the segment by a well-sclerotized connection (in lateral aspect). Segment IX (Figure 5) with very long processus basimeris ventralis appears longer due to lack of setae on posterior-margin of basimere.
 |
Figures 4–10. Ectinorus (Ectinorus) insignis Beaucournu & González-Acuña n. sp. Holotype, 4: sternum VIII; 5: segment IX; 6: processus telomeris; 7: phallosome apex – Allotype; 8: sternum VII; 9: unciform sclerotization, spermatheca and ducti.; 10: anal stylet. |
Processus basimeris or Basimere: anterior and posterior edges subequal. About 10 thin setae on anterior and external borders. Margin of posterior border with one row of nine setae (slightly thicker than the drawing). Below this row, border is hyaline and naked. Inferior margin with thin setae. Ventral margin of manubrium forms a straight line visible but not as marked as in E. ineptus. Processus basimeris ventralis narrow, relatively long, with an acuminate apex; apex is anteriorly folded up as in a penknife. External part with three long and six shorter setae on its lateral margin. Internal part with only small setae. Dorsal margin of p.b.v., with two long setae; most anterior seta at base of this process
Processus telomeris (Figure 6) or Telomere, entirely masked by basimere, not extending beyond basimere as in simonsi and nomisis. Telomere longer than broad with triangular apex posterior end forming a short forward process.
Sternum IX (Figure 5): Proximal arm broad basally, narrower ventrally; distal arm narrow, then broader with a pointed apex. One short median seta and seven long curved setae on ventral margin. Tuft of six to seven small thin setae on internal surface.
Phallosome (Figure 7): classical structure for this genus, and subgenus, but apico-ventral lobe particularly broad.
Abdomen (female genital segments): Tergum VIII with four to five small setae above spiracle, spiracle elongated and vermiform; laterally about 12 small setae. Unciform sclerotization visible above spermatheca, on tergum VIII, shaped like a “horseshoe” and slightly pigmented (Figures 8 and 9).
Sternum VII (Figure 8): posterior margin convex on ventral half with five long setae remote margin; no other seta. Anal stylet (Figure 10) cylindrical with one apical seta long and gently curved, and basally one very small seta. Sternum VIII longer than wide (and very different from E. ineptus in this regard).
Spermatheca (Figure 9): bulga somewhat quadrangular; area cribriformis in the middle of proximal end; hilla broad and subrectangular. Ductus bursae with shape of a cursive ε, characteristic of this species-group, for example in ineptus, convexus, hirsutus, etc.
Dimensions (slide-mounted specimens): holotype male 1.9 mm; allotype female 2.7 mm.
Diagnosis
Among the species of Ectinorus sensu stricto, E. ineptus Johnson, 1957 [19], E. hirsutus Hastriter, 2009 [15] and E. splendidus Smit, 1968 [34] show some similarities with E. insignis n. sp. These species belong to Ectinorus having marginal spinelets on tergum I, they also belong to the hapalus-group having processus basimeris ventralis. This group shares common characters with the former genus Dysmicus and the genus Ectinorus sensu stricto (Smit, 1987) [36].
A non-genital difference is noted for the first time. Ectinorus are split into two groups by Smit [36] with respect to whether or not they have spinelets on the margin of tergum I. As we have stated, E. insignis n. sp. belongs to the hapalus-group, having processus basimeris ventralis, but unlike ineptus and hirsutus the male has no spinelet on tergum I and the female has only one or two instead of the more typical, four or six. Indeed, Dysmicus Jordan, 1942 [21] is characterized by no spinelet on tergum I, seems to mirror Ectinorus, in this respect.
Segment IX: Basimere has the same general shape as in the taxa previously mentioned. However in insignis it is less compact; the posterior margin and chaetotaxy are also different. E. splendidus, hirsutus and insignis have almost the same processus basimeris ventralis (pbv), but in E. insignis it is straight and not bent at right angles as in E. hirsutus. The pbv is short, massive and inserted at the base of basimere in E. ineptus. Among E. Ectinorus, only E. splendidus and E. insignis share-related shapes of the pbv. Sternum IX is close to that of E. ineptus by its pointed apex. The lobe of sternum VIII is not present in E. splendidus. This lobe is almost circular in E. insignis and in E. ineptus. However it bears only two setae in E. insignis while there are eight or nine in E. ineptus (six or eight in our specimens); E. hirsutus also has a circular ventrally indented lobe. For E. insignis, sternum VII only has five setae adjacent from the margin, whereas there are 10–11 setae (11 and 12 in our specimens) close to the margin in E. ineptus and E. hirsutus. The sternum of E. splendidus is very different.
Females are very similar to each other (the female of E. splendidus is currently unknown) and always have unciform sclerotization.
Host specificity: At this time, Ectinorus insignis n. sp. is only known from Eligmodontia puerulus, a sigmodontine rodent, which is also a secondary host of Ectinorus ineptus, as cited above.
Unciform sclerotization
These are “sclerotised fold (or folds) on the anterior portion of tergum VIII of the female in some fleas, usually situated under sternum VII and near its margin” (Rothschild & Traub, 1971) [31], studied also by Smit (1970) [35] and Peus (1976) [30]. They were first drawn by Jordan (1936) [20] with the description of Ctenophthalmus singularis (presently C. (Ethioctenophthalmus) singularis), then quoted by Smit (1963) [33] in a “Species-group in Ctenophthalmus” and quoted again by Hopkins & Rothschild (1966) [17] in volume IV of “the Catalogue of the Rothschild collection of Fleas (Hystrichopsyllidae: Ctenophthalminae)” (now Ctenophthalmidae) and finally thoroughly described by Peus (op. cit.) always in various Ctenophthalmus Kolenati, 1856: as in the subgenera Ethioctenophthalmus Hopkins & Rothschild, 1966 (Figure 11), Euctenophthalmus Wagner, 1940 (Figure 12) and Metactenophthalmus Peus, 1976. Among Ctenophthalmus, on one side, the “sclerotisations”, as spelled by Rothschild & Traub (1971) [31], are rarely described in Ethioctenophthalmus a highly polymorphic subgenus which is undoubtedly polyphyletic, on the other hand, they are obvious in C. (E.) leptodactylus Hubbard, 1963, more discreet in C. (E.) smithersi De Meillon, 1952. However, they are always seen in Euctenophthalmus and their relatives in Metactenophthalmus.
 |
Figures 11–25 Unciforms sclerotizations in various species (the drawing of the outline of the spermathaeca is also included for scale and site of sclerotization) – Ctenophthalmidae, Ctenophthalminae: 11: Ctenophthalmus (Ethioctenophthalmus) eximius Jordan & Rothschild, 1913, Tanzania; 12: C. (Euctenophthalmus) savii calabricus Beaucournu, Valle & Launay, 1981, Italia – Malacopsyllidae: 13: Malacopsylla grossiventris (Weyenbergh, 1879), Argentina; 14: Phthiropsylla agenoris (Rothschild, 1904), Argentina – Rhopalopsyllidae, Parapsyllinae: 15: Delostichus coxalis (Rothschild, 1909), Chile; 16: Tetrapsyllus (Tetrapsyllus) maulinus Beaucournu & Gallardo, 1978, Chile; 17: Tetrapsyllus (Heteropsyllus) satyrus Beaucournu & Torres-Mura, 1986, Chile; 18: Listronius plesiomorphus Beaucournu & Gallardo, 1991, Chile; 19: Parapsyllus senellarti Beaucournu & Rodhain, 1990, Amsterdam Island; 20: Parapsyllus nestoris antichthones Smit, 1979, Antipodes Island – Rhopalopsyllinae: 21: Rhopalopsyllus lugubris lugubris Jordan & Rothschild, 1908, French Guyana; 22: Tiamastus helicis Beaucournu & Carmen Castro, 2003, Argentina; 23: Polygenis (Gephyropsylla) klagesi klagesi (Rothschild, 1904), French Guyana; 24: Polygenis (Polygenis) rimatus (Jordan, 1932), Brasil; 25: Polygenis (Neopolygenis) pradoi (Wagner, 1937), Colombia. |
As Peus [30] showed, these sclerotizations are complex. Depending on the species or genus, the structures will be more or less visible. So the large differences noted in forms are not surprising, for example between Ctenophthalmidae and Malacopsylloidea. This structure is also present in a number of other Families: for instance, slerotization is visible in two widely distributed species, Leptopsylla segnis (Schönherr, 1811) and Nosopsyllus fasciatus (Bosc d’Antic, 1800), Ceratophyllidae (respectively Leptopsyllinae and Ceratophyllinae) and shows similarities with those of Rhopalopsyllinae (Beaucournu, unpublished).
In the Superfamily Malacopsylloidea, even though they are consistent in a given species, unciform sclerotizations are random with respect to genus or other taxa. Their shape varies from one species or genus to another. In our opinion, the interest is not only in their shape but also their presence or absence. In the genus Ectinorus, Smit (1987) [36] draws an unciform sclerotization, without comment, for some species such as E. levipes (Jordan & Rothschild, 1923), E. viscachae (Wagner, 1937) (e.g., Ectinorus) and E. onychius (Jordan & Rothschild, 1923) (s. g. Ichyonus). But, this sclerotization is also visible in Ectinorus barrerai Jordan, 1939, E chilensis Lewis, 1976, E. cocyti (Rothschild, 1904), E. ixanus (Jordan, 1942), E. martini Lewis, 1976 and E. setosicornis Jordan, 1942. However, Smit [36] seems to have overlooked this structure when he studied these taxa. Thereafter, some species with the sclerotization were described after the publication of Smit’s “Monograph”. In E. uspallatae Beaucournu & Gallardo, 2005 [6], for example, reported it as a lacuna, a name proposed by Peus [30] for some of these structures. Conversely, it is lacking in E. (Panallius) galeanus (Jordan, 1939).
Unciform sclerotization exists in other Rhopalopsyllidae. Smit [36] drew it, for example, in various Parapsyllus and in Malacopsyllidae. This family is composed of two monotypic genera, Malacopsylla Weyenberg, 1879 and Phthiropsylla Wagner, 1939. Rhopalopsyllidae are relatives of Malacopsyllidae and form with them the Superfamily Malacopsylloidea.
Smit (1987) [35] and various authors (including Johnson, 1957 [19], Linardi & Guimarães, 1993 [26], 2000 [27]) have drawn them in Rhopalopsyllidae. But the discussion must extend beyond these drawings, because the authors did not seem interested in the structure. However, Linardi & Guimarães (2000) [27] point out “mancha escura” (dark spot) or “mancha clara escamiforme” (scaly bright spot) in several Rhopalopsyllinae. Those are various unciform sclerotizations.
Combining these data and ours, we report unciform sclerotization for Malacopsylloidea in Table 1.
Presence or absence of unciform sclerotization in the superfamily Malacopsylloidea.
In conclusion, there is unciform sclerotization for the two genera of Malacopsyllidae. In Rhopalopsyllidae – Parapsyllinae, it is present for every species of Delostichus, Tetrapsyllus, Ectinorus, Eritranis and Listronius studied. However, curiously, the enigmatic Ectinorus (Panallius) galeanus does not have it. Finally, it seems to be present in all species and subspecies of the subfamily Rhopalopsyllinae in the Rhopalopsyllidae. This structure varies in size and shape depending on the species but its presence can help species classification. We did not aim to search for the slerotization in all Siphonaptera, but hopefully by this note, we will draw attention to this structure by other researchers. Indeed the structure appears to have taxonomic value in the classification of particular subgenera within the genus Ctenophthalmus. Females, for example in Rhopalopsyllidae, are difficult to identify. Therefore, the presence or absence of sclerotization must be well figured or at least reported to aid in identification.
Acknowledgments
Collection of Chilean fleas was carried out as part as the FONDECYT 1100695 project. Thanks also to authorization of Servicio Agrícola y Ganadero and to Corporación Nacional Forestal (CONAF) for the use of their facilities in National parks and reserves.
References
- Beaucournu J-C. 2002. Heteropsyllus n. subgen. pour Tetrapsyllus satyrus Beaucournu & Torres-Mura, 1986 (Siphon., Rhopalopsyllidae). Bulletin de la Société entomologique de France, 107, 264. [Google Scholar]
- Beaucournu J-C, Carmen Castro D del. 2002. Ectinorus pilosus n. sp., nouvelle puce d’Argentine (Siphonaptera, Rhopalopsyllidae). Bulletin de la Société entomologique de France, 107, 367–369. [Google Scholar]
- Beaucournu J-C, Carmen Castro D del. 2003. Deux nouveaux Tiamastus Jordan, 1939, d’Argentine (Siphonaptera, Rhopalopsyllidae). Bulletin de la Société entomologique de France, 108, 49–53. [Google Scholar]
- Beaucournu J-C, Gallardo MH. 1989. Contribution à la faune du Chili. Puces nouvelles de la moitié nord (Siphonaptera). Bulletin de la Société entomologique de France, 94, 181–188. [Google Scholar]
- Beaucournu J-C, Gallardo MH. 1991. Siphonaptères du Chili; description de quatre espèces nouvelles (Siphonaptera). Bulletin de la Société entomologique de France, 96, 185–203. [Google Scholar]
- Beaucournu J-C, Gallardo MH. 2005. Deux puces nouvelles (Siphonaptera: Rhopalopsyllidae: Parapsyllinae) d’Argentine, parasites d’Abrocoma uspallata Braun et Mares, 2002 (Rod.: Abrocomidae). Parasite, 12, 39–43. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Beaucournu J-C, Gallardo MH, Ménier K. 2004. Deux puces nouvelles du Chili et d’Argentine (Insecta – Siphonaptera: Stephanocircidae et Rhopalopsyllidae) et érection d’un sous-genre chez Plocopsylla Jordan, 1931. Parasite, 11, 249–252. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Beaucournu J-C, González D. 2005. Ectinorus (E.) lagidium n. sp., nouveau parasite de la Viscache, Lagidium viscacia (Molina, 1782) (Rodentia, Chinchillidae), au Chili (Siphonaptera, Rhopalopsyllidae). Bulletin de la Société entomologique de France, 110, 399–401. [Google Scholar]
- Beaucournu J-C, Kelt DA. 1990. Contribution à la faune du Chili: puces nouvelles ou peu connues de la partie sud (Insecta, Siphonaptera). Revue suisse de Zoologie, 97, 647–668. [Google Scholar]
- Beaucournu J-C, Moreno L, González-Acuña D. 2011. Deux espèces nouvelles de Puces (Siphonaptera: Ctenophthalmidae & Rhopalopsyllidae) du Chili. Parasite, 18, 241–246. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Beaucournu J-C, Torres-Mura JC. 1986. Un Tetrapsyllus nouveau du Chili (Siphonaptera, Rhopalopsyllidae). Revue française d’Entomologie, (N. S.), 8, 9–12. [Google Scholar]
- Beaucournu J-C, Torres-Mura JC. 1987. Un nouveau Delostichus (Siphonaptera, Rhopalopsyllidae) d’Argentine. Bulletin de la Société Française de Parasitologie, 5, 257–260. [Google Scholar]
- Guimarães LR. 1940. Notas sôbre Siphonaptera e redescrição de Polygenis occidentalis (Almeida Cunha, 1914). Archivos de Zoologia, Sao Paulo, 2, 215–250. [Google Scholar]
- Hastriter MW. 2001. Fleas (Siphonaptera: Ctenophthalmidae and Rhopalopsyllidae) from Argentina and Chile with two new species from the rock rat, Aconaemys fuscus, in Chile. Annals of Carnegie Museum, 70, 169–178. [Google Scholar]
- Hastriter MW. 2009. A description of four new species of fleas (Insecta, Siphonaptera) from Angola, Ethiopia, Papua New Guinea, and Peru. ZooKeys, 8, 39–61. [CrossRef] [Google Scholar]
- Hastriter MW, Sage RD. 2009. A description of two new species of Ectinorus (Siphonaptera: Rhopalopsyllidae) from Laguna Blanca National Park, Neuquèn Province, Argentina. Proceedings of the Entomological Society of Washington, 111, 581–597. [CrossRef] [Google Scholar]
- Hopkins GHE, Rothschild M. 1966. An illustrated Catalogue of the Rothschild Collection of Fleas. Vol. IV: Hystrichopsyllidae (Ctenophthalminae, Dinopsyllinae, Doratopsyllinae and Listropsyllinae). Trustees of the British Museum (Natural History), London–549 pp. [Google Scholar]
- Jameson EW, Fulk GW. 1977. Notes on some fleas (Siphonaptera) from Chile. Journal of Medical Entomology, 14, 401–406. [PubMed] [Google Scholar]
- Jordan K. 1957. A classification of the Siphonaptera of South America, with descriptions of new species. Memoirs of the Entomological Society of Washington, vol. 5, 298 pp. [Google Scholar]
- Jordan K. 1936. Siphonaptera from Congo belge. Novitates Zoologicae, 39, 294–299. [Google Scholar]
- Jordan K. 1942. On Parapsyllus and some closely related genera of Siphonaptera. Eos. Revista española de Entomologia, 18, 7–29. [Google Scholar]
- Jordan K. 1942. On the Siphonaptera collected by Dr. J. M. de la Barrera in the provinces of Mendoza during 1939. Revista del Instituto Bacteriologico « Dr. Carlos G. Malbran », 10, 401–460. [Google Scholar]
- Jordan K, Rothschild NC. 1908. Revision of the non-combed eyed Siphonaptera. Parasitology, 1, 1–100. [CrossRef] [Google Scholar]
- Jordan K, Rothschild NC. 1923. On the genera Rhopalopsyllus and Parapsyllus. Ectoparasites, 1, 320–370. [Google Scholar]
- Lewis RL. 1976. A review of the South-American flea subgenus Ectinorus Jordan 1942, with descriptions of two new species and a key (Siphonaptera: Rhopalopsyllidae). Journal of Parasitology, 62, 1003–1009. [CrossRef] [Google Scholar]
- Linardi PM, Guimarães LR. 1993. Systematic review of genera and subgenera of Rhopalopsyllinae (Siphonaptera: Rhopalopsyllidae) by phenetic and cladistic methods. Journal of Medical Entomology, 30, 161–170. [PubMed] [Google Scholar]
- Linardi PM, Guimarães LR. 2000. Sifonápteros do Brasil. Museo de Zoologia, USP, FAPESP, São Paulo, vol. 5, 291 pp. [Google Scholar]
- Macchiavello A. 1948. Sifonaptera de la Costa Sur-Occidental de America (Primera lista y Distribucion Zoo-Geografica). Boletin de la Oficina Sanitaria Panamericana, 27, 412–460. [Google Scholar]
- Méndez E. 1968. Scolopsyllus colombianus, new genus and new species of the family Rhopalopsyllidae (Siphonaptera) from Colombia. Journal of Medical Entomology, 5, 405–410. [PubMed] [Google Scholar]
- Peus F. 1976. Flöhe aus Anatolien und anderen Ländern des Nahen Ostens. Abhandlungen der Zoologisch-Botanischen Gesellschaft in Wien, 20, 1–111. [Google Scholar]
- Rothschild M, Traub R. 1971. A revised Glossary of terms used in the taxonomy and morphology of Fleas. An Illustrated Catalogue of the Rothschild Collection of Fleas (Siphonaptera) in the British Museum (Natural History), vol. V, pp. 8–85. [Google Scholar]
- Rothschild NC. 1904. Further contributions to the knowledge of the Siphonaptera. Novitates Zoologicae, 11, 602–653. [Google Scholar]
- Smit FGAM. 1963. Species-groups in Ctenophthalmus (Siphonaptera: Hystrichopsyllidae). Bulletin of the British Museum (Natural History), Entomology, 14, 107–152. [Google Scholar]
- Smit FGAM. 1968. Siphonaptera taken from formalin-traps in Chile. Zoologischer Anzeiger, 180, 220–228. [Google Scholar]
- Smit FGAM. 1970. Siphonaptera. Tuxen S.L.: Taxonomist’s glossary of genitalia in insects. Second revised and enlarged edition, Munksgaard, Copenhagen, p. 141–156. [Google Scholar]
- Smit FGAM. 1987. An illustrated catalogue of the Rothschild collection of Fleas Vol. VII: Malacopsylloidea (Malacopsyllidae and Rhopalopsyllidae). Oxford University Press, The British Museum (Natural History), Oxford & London, 380 p. [Google Scholar]
- Wilson DE, Reeder DM. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd edn. The Johns Hopkins University Press: Baltimore. [Google Scholar]
Cite this article as: Beaucournu J-C, Belaz S, Muñoz-Léal S & González-Acuña D: A new flea,Ectinorus (Ectinorus) insignis n. sp. (Siphonaptera, Rhopalopsyllidae, Parapsyllinae), with notes on the subgenus Ectinorus in Chile and comments on unciform sclerotization in the superfamily Malacopsylloidea. Parasite, 2013, 20, 35.
All Tables
Presence or absence of unciform sclerotization in the superfamily Malacopsylloidea.
All Figures
 |
Figures 1–3. Ectinorus (Ectinorus) ineptus (Johnson, 1957) neallotype. 1: anal stylet; 2: terminal segment and unciform sclerotization; 3: spermatheca and ducti. |
| In the text | |
 |
Figures 4–10. Ectinorus (Ectinorus) insignis Beaucournu & González-Acuña n. sp. Holotype, 4: sternum VIII; 5: segment IX; 6: processus telomeris; 7: phallosome apex – Allotype; 8: sternum VII; 9: unciform sclerotization, spermatheca and ducti.; 10: anal stylet. |
| In the text | |
 |
Figures 11–25 Unciforms sclerotizations in various species (the drawing of the outline of the spermathaeca is also included for scale and site of sclerotization) – Ctenophthalmidae, Ctenophthalminae: 11: Ctenophthalmus (Ethioctenophthalmus) eximius Jordan & Rothschild, 1913, Tanzania; 12: C. (Euctenophthalmus) savii calabricus Beaucournu, Valle & Launay, 1981, Italia – Malacopsyllidae: 13: Malacopsylla grossiventris (Weyenbergh, 1879), Argentina; 14: Phthiropsylla agenoris (Rothschild, 1904), Argentina – Rhopalopsyllidae, Parapsyllinae: 15: Delostichus coxalis (Rothschild, 1909), Chile; 16: Tetrapsyllus (Tetrapsyllus) maulinus Beaucournu & Gallardo, 1978, Chile; 17: Tetrapsyllus (Heteropsyllus) satyrus Beaucournu & Torres-Mura, 1986, Chile; 18: Listronius plesiomorphus Beaucournu & Gallardo, 1991, Chile; 19: Parapsyllus senellarti Beaucournu & Rodhain, 1990, Amsterdam Island; 20: Parapsyllus nestoris antichthones Smit, 1979, Antipodes Island – Rhopalopsyllinae: 21: Rhopalopsyllus lugubris lugubris Jordan & Rothschild, 1908, French Guyana; 22: Tiamastus helicis Beaucournu & Carmen Castro, 2003, Argentina; 23: Polygenis (Gephyropsylla) klagesi klagesi (Rothschild, 1904), French Guyana; 24: Polygenis (Polygenis) rimatus (Jordan, 1932), Brasil; 25: Polygenis (Neopolygenis) pradoi (Wagner, 1937), Colombia. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


